Nicotine - Addiction/Dependence: Difference between revisions
| (25 intermediate revisions by the same user not shown) | |||
| Line 7: | Line 7: | ||
''' <big><span style="background-color: rgb(255, 255, 0);" data-mce-style="background-color: #ffff00;">[https://pubmed.ncbi.nlm.nih.gov/35302289/ Abuse Liability]</span>: The potential to develop a dependence or addiction to a substance.</big>''' | ''' <big><span style="background-color: rgb(255, 255, 0);" data-mce-style="background-color: #ffff00;">[https://pubmed.ncbi.nlm.nih.gov/35302289/ Abuse Liability]</span>: The potential to develop a dependence or addiction to a substance.</big>''' | ||
'''<big><span style="background-color: rgb(255, 255, 0);" data-mce-style="background-color: #ffff00;">Recommended Podcast</span>: [https://www.thestudiesshowpod.com/p/episode-40-addiction The Studies Show Episode 40: Addiction]</big>''' | |||
'''<big><span style="background-color: rgb(255, 255, 0);" data-mce-style="background-color: #ffff00;">Recommended Video</span>: [https://www.youtube.com/watch?v=Xwi-nRS4kjU Tobacco Addiction & Nicotine Dependency] | |||
<br> | <br> | ||
='''Background Information'''= | |||
===2011 [https://research.med.psu.edu/smoking/dependence-index/ Penn State Nicotine Dependence Index]=== | |||
*The Penn State Nicotine Dependence Index was developed by Dr. Jonathan Foulds in 2011. This 10-item scale (with scores ranging from 0 to 20) was developed to measure nicotine dependence across all nicotine product types, and an adapted version was the first dependence measure designed to evaluate electronic cigarette dependence. | |||
===2009 [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2798587/ Abuse liability assessment of tobacco products including potential reduced exposure products]=== | |||
*In the 2009 law giving FDA regulation over tobacco products, FDA is now required to evaluate new tobacco products including MRTP/PREPs to determine their risk for abuse and toxicity at the population level. This article describes the traditional tools and methods of ALA that can be used to evaluate new tobacco and nicotine products including MRTP/PREPs. Such ALA data could contribute to the scientific foundation on which future public policy decisions are based. | |||
===2003 [https://www.nature.com/articles/1300030 A Self-Administered Questionnaire to Measure Dependence on Cigarettes: The Cigarette Dependence Scale]=== | |||
*The aim of this study was to develop a new, self-administered measure of cigarette dependence. | |||
===1991 [https://cde.nida.nih.gov/instrument/d7c0b0f5-b865-e4de-e040-bb89ad43202b Fagerstrom Test for Nicotine Dependence]=== | |||
*The Fagerström Test for Nicotine Dependence is a standard instrument for assessing the intensity of physical addiction to nicotine. The test was designed to provide an ordinal measure of nicotine dependence related to cigarette smoking. It contains six items that evaluate the quantity of cigarette consumption, the compulsion to use, and dependence. | |||
='''Smoking - Combustible Tobacco'''= | ='''Smoking - Combustible Tobacco'''= | ||
| Line 27: | Line 45: | ||
***Acknowledgment: Supported by grants from the Flight Attendants Medical Research Institute and the National Institute on Drug Abuse (U.S. Public Health Service grants DA02277 and DA20830). | ***Acknowledgment: Supported by grants from the Flight Attendants Medical Research Institute and the National Institute on Drug Abuse (U.S. Public Health Service grants DA02277 and DA20830). | ||
='''ENDS/E-Cigarettes/NVP/Vaping'''= | ='''ENDS/E-Cigarettes/NVP/Vaping vs Smoking Tobacco'''= | ||
*ENDS = Electronic Nicotine Delivery System | *ENDS = Electronic Nicotine Delivery System | ||
*NVP = Nicotine Vapor Product | *NVP = Nicotine Vapor Product | ||
===2023 [https://pubmed.ncbi.nlm.nih.gov/36994368/ Changes in dependence over one year among US adults who smoke cigarettes and switched completely or partially to use of the JUUL-brand electronic nicotine delivery system]=== | ===2023 [https://pubmed.ncbi.nlm.nih.gov/36994368/ Changes in dependence over one year among US adults who smoke cigarettes and switched completely or partially to use of the JUUL-brand electronic nicotine delivery system]=== | ||
| Line 45: | Line 58: | ||
**Citation: Jones DM, Guy MC, Fairman BJ, Soule E, Eissenberg T, Fagan P. Nicotine Dependence among Current Cigarette Smokers Who Use E-Cigarettes and Cannabis. Subst Use Misuse. 2023;58(5):618-628. doi: 10.1080/10826084.2023.2177961. Epub 2023 Feb 27. PMID: 36852436; PMCID: PMC10249428. | **Citation: Jones DM, Guy MC, Fairman BJ, Soule E, Eissenberg T, Fagan P. Nicotine Dependence among Current Cigarette Smokers Who Use E-Cigarettes and Cannabis. Subst Use Misuse. 2023;58(5):618-628. doi: 10.1080/10826084.2023.2177961. Epub 2023 Feb 27. PMID: 36852436; PMCID: PMC10249428. | ||
***Acknowledgment: Funding; K01 DA055088/DA/NIDA NIH HHS/United States and U54 DA036105/DA/NIDA NIH HHS/United States. COI; Thomas Eissenberg is a paid consultant in litigation against the tobacco industry and also the electronic cigarette industry and is named on one patent for a device that measures the puffing behavior of electronic cigarette users, on another patent application for a smartphone app that determines electronic cigarette device and liquid characteristics, and a third patent application for a smoking cessation intervention. Eric Soule is named on a patent application for a smartphone app that determines electronic cigarette device and liquid characteristics. The other authors declare they have no conflicts of interest. | ***Acknowledgment: Funding; K01 DA055088/DA/NIDA NIH HHS/United States and U54 DA036105/DA/NIDA NIH HHS/United States. COI; Thomas Eissenberg is a paid consultant in litigation against the tobacco industry and also the electronic cigarette industry and is named on one patent for a device that measures the puffing behavior of electronic cigarette users, on another patent application for a smartphone app that determines electronic cigarette device and liquid characteristics, and a third patent application for a smoking cessation intervention. Eric Soule is named on a patent application for a smartphone app that determines electronic cigarette device and liquid characteristics. The other authors declare they have no conflicts of interest. | ||
===2022 [https://pubmed.ncbi.nlm.nih.gov/36543869/ Part one: abuse liability of Vuse Solo (G2) electronic nicotine delivery system relative to combustible cigarettes and nicotine gum]=== | ===2022 [https://pubmed.ncbi.nlm.nih.gov/36543869/ Part one: abuse liability of Vuse Solo (G2) electronic nicotine delivery system relative to combustible cigarettes and nicotine gum]=== | ||
| Line 63: | Line 71: | ||
===2021 [https://onlinelibrary.wiley.com/doi/10.1111/add.15403 Dependence on nicotine in US high school students in the context of changing patterns of tobacco product use]=== | ===2021 [https://onlinelibrary.wiley.com/doi/10.1111/add.15403 Dependence on nicotine in US high school students in the context of changing patterns of tobacco product use]=== | ||
*Among US high school students, increases in the prevalence of nicotine product use from 2012 to 2019 do not appear to have been accompanied by a similar increase in the population burden of nicotine dependence. This may be at least partly attributable to a shift in the most common product of choice from cigarettes (on which users are most dependent) to e-cigarettes (on which users are least dependent). | *Among US high school students, increases in the prevalence of nicotine product use from 2012 to 2019 do not appear to have been accompanied by a similar increase in the population burden of nicotine dependence. This may be at least partly attributable to a shift in the most common product of choice from cigarettes (on which users are most dependent) to e-cigarettes (on which users are least dependent). | ||
*Use of e-cigarettes increased dramatically, use of cigarettes declined, and use of combustible (non-cigarette) and smokeless tobacco was relatively stable. Whether the overall increase in product use has been mirrored by an increase in nicotine dependence was unclear. | |||
*We found that different tobacco products were associated with differing levels of nicotine dependence, with cigarettes characterised by highest dependence and e-cigarettes in otherwise tobacco-naïve students by low dependence. | |||
*The increase in population use of tobacco products between 2012 and 2019 (from 23.2% to 31.2%) was not accompanied by an equivalent increase in overall population burden of dependence (craving: 10.9% to 9.5%; wanting to use within 30min: 4.7% to 5.4%). | |||
*[https://onlinelibrary.wiley.com/doi/epdf/10.1111/add.15403 PDF Version] | *[https://onlinelibrary.wiley.com/doi/epdf/10.1111/add.15403 PDF Version] | ||
*[[File:Youth_Dependence.jpg|Youth Dependence on Nicotine Products]] | |||
**Citation: Jackson, S. E., Brown, J., and Jarvis, M. J. (2021) Dependence on nicotine in US high school students in the context of changing patterns of tobacco product use. Addiction, 116: 1859– 1870. doi: 10.1111/add.15403 | **Citation: Jackson, S. E., Brown, J., and Jarvis, M. J. (2021) Dependence on nicotine in US high school students in the context of changing patterns of tobacco product use. Addiction, 116: 1859– 1870. doi: 10.1111/add.15403 | ||
***Acknowledgement: Cancer Research UK (C1417/A22962) supported S.J. andJ.B.’s salaries. | ***Acknowledgement: Cancer Research UK (C1417/A22962) supported S.J. andJ.B.’s salaries. | ||
===2021 [https://pubmed.ncbi.nlm.nih.gov/33894800/ Changes in Dependence as Smokers Switch from Cigarettes to JUUL in Two Nicotine Concentrations]=== | ===2021 [https://pubmed.ncbi.nlm.nih.gov/33894800/ Changes in Dependence as Smokers Switch from Cigarettes to JUUL in Two Nicotine Concentrations]=== | ||
| Line 107: | Line 119: | ||
***Acknowledgment: Grants from the National Cancer Institute of the National Institutes of Health (P20CA202921 to University of Oklahoma, and 5P20CA202923 to Cherokee Nation) supported this study. The funding body had no role in the design of the study and collection, analysis, interpretation of data, or writing the manuscript. Content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health or of Cherokee Nation. The authors declare that they have no competing interests. | ***Acknowledgment: Grants from the National Cancer Institute of the National Institutes of Health (P20CA202921 to University of Oklahoma, and 5P20CA202923 to Cherokee Nation) supported this study. The funding body had no role in the design of the study and collection, analysis, interpretation of data, or writing the manuscript. Content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health or of Cherokee Nation. The authors declare that they have no competing interests. | ||
===2019 [https:// | ===2019 [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6754311/ Abuse liability assessment of an electronic cigarette in combustible cigarette smokers]=== | ||
*" | *"Collectively, the results of this study demonstrated that the ECIG device and liquids examined had moderate levels of abuse liability: on average lower than combustible cigarettes, but higher than an FDA-approved nicotine replacement therapy (i.e., nicotine inhaler)." | ||
**Citation: Maloney SF, Breland A, Soule EK, Hiler M, Ramôa C, Lipato T, Eissenberg T. Abuse liability assessment of an electronic cigarette in combustible cigarette smokers. Exp Clin Psychopharmacol. 2019 Oct;27(5):443-454. doi: 10.1037/pha0000261. Epub 2019 Feb 18. PMID: 30777773; PMCID: PMC6754311. | |||
**Citation: | ***Acknowledgment: Funding; This study was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number P50DA036105 and U54DA036105 and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration. COI; Dr. Eissenberg is a paid consultant in litigation against the tobacco industry and is named on a patent for a device that measures the puffing behavior of ECIG users. | ||
***Acknowledgment: This | |||
===2018 [https://pubmed.ncbi.nlm.nih.gov/29725702/ Assessment of the abuse liability of three menthol Vuse Solo electronic cigarettes relative to combustible cigarettes and nicotine gum]=== | ===2018 [https://pubmed.ncbi.nlm.nih.gov/29725702/ Assessment of the abuse liability of three menthol Vuse Solo electronic cigarettes relative to combustible cigarettes and nicotine gum]=== | ||
| Line 118: | Line 129: | ||
**Citation: Stiles MF, Campbell LR, Jin T, Graff DW, Fant RV, Henningfield JE. Assessment of the abuse liability of three menthol Vuse Solo electronic cigarettes relative to combustible cigarettes and nicotine gum. Psychopharmacology (Berl). 2018 Jul;235(7):2077-2086. doi: 10.1007/s00213-018-4904-x. Epub 2018 May 3. PMID: 29725702; PMCID: PMC6015619. | **Citation: Stiles MF, Campbell LR, Jin T, Graff DW, Fant RV, Henningfield JE. Assessment of the abuse liability of three menthol Vuse Solo electronic cigarettes relative to combustible cigarettes and nicotine gum. Psychopharmacology (Berl). 2018 Jul;235(7):2077-2086. doi: 10.1007/s00213-018-4904-x. Epub 2018 May 3. PMID: 29725702; PMCID: PMC6015619. | ||
***Acknowledgement: ClinicalTrials.gov identifier: NCT02664012. MF Stiles, LR Campbell, and T Jin are full-time employees of RAI Services Company. RAI Services Company is a wholly owned subsidiary of Reynolds American Inc., which is a wholly owned subsidiary of British American Tobacco plc. DW Graff is a full-time employee of Celerion and provided the original draft of the manuscript. RV Fant and JE Henningfield are full-time employees of PinneyAssociates, which provides consulting services on smoking cessation and tobacco harm minimization (including nicotine replacement therapy and electronic vapor products) to Niconovum, USA, Inc., RJ Reynolds Vapor Company, and RAI Services Company (all subsidiaries of Reynolds American Inc.). JE Henningfield also owns an interest in intellectual property for a novel nicotine medication. Through PinneyAssociates, Fant and Henningfield provide consulting services to pharmaceutical companies on abuse potential assessment, and the regulation of substances with a potential for abuse. | ***Acknowledgement: ClinicalTrials.gov identifier: NCT02664012. MF Stiles, LR Campbell, and T Jin are full-time employees of RAI Services Company. RAI Services Company is a wholly owned subsidiary of Reynolds American Inc., which is a wholly owned subsidiary of British American Tobacco plc. DW Graff is a full-time employee of Celerion and provided the original draft of the manuscript. RV Fant and JE Henningfield are full-time employees of PinneyAssociates, which provides consulting services on smoking cessation and tobacco harm minimization (including nicotine replacement therapy and electronic vapor products) to Niconovum, USA, Inc., RJ Reynolds Vapor Company, and RAI Services Company (all subsidiaries of Reynolds American Inc.). JE Henningfield also owns an interest in intellectual property for a novel nicotine medication. Through PinneyAssociates, Fant and Henningfield provide consulting services to pharmaceutical companies on abuse potential assessment, and the regulation of substances with a potential for abuse. | ||
===2018 [https://www.sciencedirect.com/science/article/abs/pii/S0306460317302885 Then and now: Consumption and dependence in e-cigarette users who formerly smoked cigarettes]=== | |||
*Conclusion: "First, we found that the large majority of vapers were ex-smokers who had either ceased or dramatically reduced their cigarette consumption. Second, there was a marked decrease in dependence among vapers compared to their retrospective prior cigarette dependence..." | |||
*[https://sci-hub.st/10.1016/j.addbeh.2017.07.034 PDF of full study] | |||
**Citation: Browne M, Todd DG. Then and now: Consumption and dependence in e-cigarette users who formerly smoked cigarettes. Addict Behav. 2018 Jan;76:113-121. doi: 10.1016/j.addbeh.2017.07.034. Epub 2017 Jul 28. PMID: 28780356. | |||
***Acknowledgement: Research was supported by block funding to the School of Health, Medical and Applied Sciences, Central Queensland University | |||
===2018: [https://pubmed.ncbi.nlm.nih.gov/29065204/ The Role of Nicotine Dependence in E-Cigarettes' Potential for Smoking Reduction]=== | |||
*"These findings offer possible support that e-cigarettes may act as a smoking reduction method among highly nicotine-dependent young adult cigarette smokers." | |||
**Citation: Selya AS, Dierker L, Rose JS, Hedeker D, Mermelstein RJ. The Role of Nicotine Dependence in E-Cigarettes' Potential for Smoking Reduction. Nicotine Tob Res. 2018 Sep 4;20(10):1272-1277. doi: 10.1093/ntr/ntx160. PMID: 29065204; PMCID: PMC6121914. | |||
***Acknowledgment: Grants and funding, P01 CA098262/CA/NCI NIH HHS/United States, P50 DA039838/DA/NIDA NIH HHS/United States | |||
===2017 [https://pubmed.ncbi.nlm.nih.gov/28389330/ A comparison of nicotine dependence among exclusive E-cigarette and cigarette users in the PATH study]=== | ===2017 [https://pubmed.ncbi.nlm.nih.gov/28389330/ A comparison of nicotine dependence among exclusive E-cigarette and cigarette users in the PATH study]=== | ||
| Line 128: | Line 150: | ||
**Citation: González Roz A, Secades Villa R, Weidberg S. Evaluating nicotine dependence levels in e-cigarette users. Adicciones. 2017 Jan 11;29(2):136-138. English, Spanish. doi: 10.20882/adicciones.905. PMID: 28170058. | **Citation: González Roz A, Secades Villa R, Weidberg S. Evaluating nicotine dependence levels in e-cigarette users. Adicciones. 2017 Jan 11;29(2):136-138. English, Spanish. doi: 10.20882/adicciones.905. PMID: 28170058. | ||
***Acknowledgment: Funding for this study was provided by the BBVA foundation (SV-14-FBBVA-1). This institution had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication. No conflicts reported. | ***Acknowledgment: Funding for this study was provided by the BBVA foundation (SV-14-FBBVA-1). This institution had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication. No conflicts reported. | ||
===2017: [https://onlinelibrary.wiley.com/doi/abs/10.1111/add.14013 Evaluating the mutual pathways among electronic cigarette use, conventional smoking and nicotine dependence]=== | |||
*Conclusion: "Nicotine dependence is not a significant mechanism for e-cigarettes’ purported effect on heavier future conventional smoking among young adults. Nicotine dependence may be a mechanism for increases in e-cigarette use among heavier conventional smokers, consistent with e-cigarettes as a smoking reduction tool. Overall, conventional smoking and, to a lesser extent, its resulting nicotine dependence, are the strongest drivers or signals of later cigarette and e-cigarette use." | |||
**Citation: Selya AS, Rose JS, Dierker L, Hedeker D, Mermelstein RJ. Evaluating the mutual pathways among electronic cigarette use, conventional smoking and nicotine dependence. Addiction. 2018 Feb;113(2):325-333. doi: 10.1111/add.14013. Epub 2017 Sep 25. PMID: 28841780; PMCID: PMC5760290. | |||
***Acknowledgment: Grants and funding, L40 DA042431/DA/NIDA NIH HHS/United States, P01 CA098262/CA/NCI NIH HHS/United States, P30 DK092949/DK/NIDDK NIH HHS/United States, P50 DA039838/DA/NIDA NIH HHS/United States | |||
===2017 [https://pubmed.ncbi.nlm.nih.gov/28780356/ Then and now: Consumption and dependence in e-cigarette users who formerly smoked cigarettes]=== | ===2017 [https://pubmed.ncbi.nlm.nih.gov/28780356/ Then and now: Consumption and dependence in e-cigarette users who formerly smoked cigarettes]=== | ||
| Line 133: | Line 160: | ||
**Citation: Browne M, Todd DG. Then and now: Consumption and dependence in e-cigarette users who formerly smoked cigarettes. Addict Behav. 2018 Jan;76:113-121. doi: 10.1016/j.addbeh.2017.07.034. Epub 2017 Jul 28. PMID: 28780356. | **Citation: Browne M, Todd DG. Then and now: Consumption and dependence in e-cigarette users who formerly smoked cigarettes. Addict Behav. 2018 Jan;76:113-121. doi: 10.1016/j.addbeh.2017.07.034. Epub 2017 Jul 28. PMID: 28780356. | ||
***Acknowledgement: Research was supported by block funding to the School of Health, Medical and Applied Sciences, Central Queensland University. No conflicts of interest to declare. | ***Acknowledgement: Research was supported by block funding to the School of Health, Medical and Applied Sciences, Central Queensland University. No conflicts of interest to declare. | ||
===2017 [https://pubmed.ncbi.nlm.nih.gov/28634710/ Pharmacodynamic and pharmacokinetic assessment of electronic cigarettes, combustible cigarettes, and nicotine gum: implications for abuse liability]=== | |||
*"In summary, this study is the most robust assessment of the abuse liability of ECs published to date and uses approaches similar to those found in classic abuse liability studies of pharmaceutical products, including multiple instruments to measure the subjective effects of product use, as well as nicotine uptake. Under the set of study conditions described herein, use of the three Vuse Solo ECs tended to result in subjective measures responses and nicotine uptake that were between those measured with use of combustible cigarettes and nicotine gum. In general, the results are consistent with the conclusions of others that the abuse liability of ECs as a category is less than that of combustible cigarettes but greater than for nicotine gum, and likely other nicotine replacement products' | |||
**Citation: Stiles MF, Campbell LR, Graff DW, Jones BA, Fant RV, Henningfield JE. Pharmacodynamic and pharmacokinetic assessment of electronic cigarettes, combustible cigarettes, and nicotine gum: implications for abuse liability. Psychopharmacology (Berl). 2017 Sep;234(17):2643-2655. doi: 10.1007/s00213-017-4665-y. Epub 2017 Jun 20. PMID: 28634710; PMCID: PMC5548902. | |||
***Acknowledgment: Funding; This study was funded by RJ Reynolds Vapor Company through its affiliate RJ Reynolds Tobacco Company. COI; MF Stiles, LR Campbell, and BA Jones are full-time employees of RAI Services Company, which provides support across the Reynolds American Inc. operating companies. DW Graff is a full-time employee of Celerion and provided the original draft of this manuscript. RV Fant and JE Henningfield are full-time employees of PinneyAssociates, which provides consulting services on tobacco harm minimization (including nicotine replacement therapy and digital vapor products) to Niconovum USA, RJ Reynolds Vapor Company, and RAI Services Company (all subsidiaries of Reynolds American Inc.) In the past 3 years, PinneyAssociates has consulted to GlaxoSmithKline Consumer Healthcare on smoking cessation and NJOY on electronic cigarettes. JE Henningfield also owns an interest in intellectual property for a novel nicotine medication, an option for which has been sold to Niconovum USA. Through PinneyAssociates, Fant and Henningfield also provide consulting services to pharmaceutical companies on abuse potential assessment and the regulation of substances with a potential for abuse. | |||
===2015 [https://pubmed.ncbi.nlm.nih.gov/25332459/ Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking E-cigarette users]=== | ===2015 [https://pubmed.ncbi.nlm.nih.gov/25332459/ Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking E-cigarette users]=== | ||
| Line 143: | Line 175: | ||
**Citation: Etter JF, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015 Feb 1;147:68-75. doi: 10.1016/j.drugalcdep.2014.12.007. Epub 2014 Dec 18. PMID: 25561385; PMCID: PMC4920051. | **Citation: Etter JF, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015 Feb 1;147:68-75. doi: 10.1016/j.drugalcdep.2014.12.007. Epub 2014 Dec 18. PMID: 25561385; PMCID: PMC4920051. | ||
***This study was partly funded by the Swiss Tobacco Prevention Fund (Swiss Federal Office of Public Health), grant 12.000189 to JFE. The Swiss Tobacco Prevention Fund had no role in the design or conduct of the study, interpretation of the data or decision to submit the paper for publication. TE is supported by the National Institute on Drug Abuse of the U.S. National Institutes of Health under Award Number P50DA036105 and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration. | ***This study was partly funded by the Swiss Tobacco Prevention Fund (Swiss Federal Office of Public Health), grant 12.000189 to JFE. The Swiss Tobacco Prevention Fund had no role in the design or conduct of the study, interpretation of the data or decision to submit the paper for publication. TE is supported by the National Institute on Drug Abuse of the U.S. National Institutes of Health under Award Number P50DA036105 and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration. | ||
===2012 [https://ncbi.nlm.nih.gov/pmc/articles/PMC3330136/ Clinical laboratory assessment of the abuse liability of an electronic cigarette]=== | |||
*Conclusion: Electronic cigarettes can deliver clinically significant amounts of nicotine and reduce cigarette abstinence symptoms and appear to have lower potential for abuse relative to traditional tobacco cigarettes, at least under certain laboratory conditions. | |||
**Citation:Vansickel AR, Weaver MF, Eissenberg T. Clinical laboratory assessment of the abuse liability of an electronic cigarette. Addiction. 2012 Aug;107(8):1493-500. doi: 10.1111/j.1360-0443.2012.03791.x. Epub 2012 May 8. PMID: 22229871; PMCID: PMC3330136. | |||
***Acknowledgment: This work was partially funded by the National Cancer Institute, National Institutes of Health RO1CA 120142 (awarded to Dr. Eissenberg). Dr. Vansickel was supported by National Institute on Drug Abuse, National Institutes of Health T32DA 007027. The authors have no conflicts of interest to declare. | |||
='''ENDS/E-Cigarettes/NVP/Vaping - Not Compared with Smoking Tobacco'''= | |||
===2024 [https://pubmed.ncbi.nlm.nih.gov/38800771/ Nicotine Dependency Levels Among Adult Electronic Cigarette Smokers in Jeddah, Saudi Arabia: An Analytical Cross-Sectional Study]=== | |||
*Conclusion: "Most exclusive EC users in the study developed a moderate nicotine dependence level. The EC device type and nicotine concentration were significant drivers of nicotine dependence." | |||
**Citation: Yahya L, Mandoura N, Harere R. Nicotine Dependency Levels Among Adult Electronic Cigarette Smokers in Jeddah, Saudi Arabia: An Analytical Cross-Sectional Study. Cureus. 2024 May 25;16(5):e61038. doi: 10.7759/cureus.61038. PMID: 38800771; PMCID: PMC11127123. | |||
***Acknowledgment: The authors have declared that no competing interests exist. (No funding mentioned.) | |||
===2022 [https://pubmed.ncbi.nlm.nih.gov/35305014/ The Role of Nicotine and Flavor in the Abuse Potential and Appeal of Electronic Cigarettes for Adult Current and Former Cigarette and Electronic Cigarette Users: A Systematic Review]=== | |||
*"Implications: E-cigarettes may provide a reduced-harm alternative to cigarettes for smokers unwilling/unable to quit or serve as a path for quitting all nicotine products. Higher nicotine concentrations and flavor variety are associated with higher abuse potential and appeal of e-cigarettes. Higher abuse potential and appeal products may help facilitate complete switching from cigarettes to e-cigarettes. Regulation of nicotine concentration and flavors aimed at decreasing naïve uptake may inadvertently decrease uptake and complete switching among smokers, reducing the harm reduction potential of e-cigarettes. Evidence-based effects of regulating nicotine concentration and flavors must be considered for the population as a whole, including smokers." | |||
**Citation: Gades MS, Alcheva A, Riegelman AL, Hatsukami DK. The Role of Nicotine and Flavor in the Abuse Potential and Appeal of Electronic Cigarettes for Adult Current and Former Cigarette and Electronic Cigarette Users: A Systematic Review. Nicotine Tob Res. 2022 Aug 6;24(9):1332-1343. doi: 10.1093/ntr/ntac073. PMID: 35305014; PMCID: PMC9356694. | |||
***Acknowledgment: This work was supported by the National Institute of Drug Abuse (T32 DA007097 and R36 DA050000 to MSG); and the National Institutes of Health (P01 CA217806 to DKH). No COI declared. | |||
===2019 [https://pubmed.ncbi.nlm.nih.gov/31375364/ Changes in E-Cigarette Use Behaviors and Dependence in Long-term E-Cigarette Users]=== | |||
*"Results: A total of 494 subjects provided complete data on both surveys. At baseline, 402 subjects (81.4%) were exclusive e-cigarette users, and 71 subjects (14.4%) were poly users. Among baseline exclusive e-cigarette users, the majority (88.3%) continued using e-cigarettes exclusively, but 37 users (9.2%) became poly users and 1 returned to cigarette smoking at follow-up. Among baseline poly users, 60.6% became exclusive e-cigarette users at follow-up. The mean PSECDI score remained similar over time (8.4 at baseline vs 8.3 at follow-up)." | |||
*NOTE: For information on the Penn State Ecigarette Dependence Index see this [https://research.med.psu.edu/smoking/dependence-index/ link]. Scores: 4 to 8 is low dependence and 9 to 12 is a medium dependence. | |||
**Citation: Du P, Fan T, Yingst J, Veldheer S, Hrabovsky S, Chen C, Foulds J. Changes in E-Cigarette Use Behaviors and Dependence in Long-term E-Cigarette Users. Am J Prev Med. 2019 Sep;57(3):374-383. doi: 10.1016/j.amepre.2019.04.021. Epub 2019 Jul 31. PMID: 31375364; PMCID: PMC9811611. | |||
***Acknowledgment: This work was supported by the National Institute on Drug Abuse of NIH and the Center for Tobacco Products of the U.S. Food and Drug Administration (P50-DA-036107). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the U.S. Food and Drug Administration. Jonathan Foulds has acted as a paid consultant for pharmaceutical companies involved in producing smoking-cessation medications, including GSK, Pfizer, Novartis, and J&J and received a research grant and study products from Pfizer Inc. No other financial disclosures were reported. | |||
===2015 [https://pubmed.ncbi.nlm.nih.gov/25592454/ Explaining the effects of electronic cigarettes on craving for tobacco in recent quitters]=== | ===2015 [https://pubmed.ncbi.nlm.nih.gov/25592454/ Explaining the effects of electronic cigarettes on craving for tobacco in recent quitters]=== | ||
| Line 148: | Line 203: | ||
**Citation: Etter JF. Explaining the effects of electronic cigarettes on craving for tobacco in recent quitters. Drug Alcohol Depend. 2015 Mar 1;148:102-8. doi: 10.1016/j.drugalcdep.2014.12.030. Epub 2015 Jan 3. PMID: 25592454. | **Citation: Etter JF. Explaining the effects of electronic cigarettes on craving for tobacco in recent quitters. Drug Alcohol Depend. 2015 Mar 1;148:102-8. doi: 10.1016/j.drugalcdep.2014.12.030. Epub 2015 Jan 3. PMID: 25592454. | ||
***Acknowledgment: This study was partly funded by the Swiss Tobacco Prevention Fund (Swiss Federal Office of Public Health), grant 12.000189 to JFE. The Swiss Tobacco Prevention Fund had no role in the design or conduct of the study, interpretation of the data or decision to submit the paper for publication... JFE was reimbursed by Dekang, a manufacturer of e-cigarettes and e-liquids for traveling to London and to China, to visit e-cigarette factories, but he received no honoraria for these meetings. JFE's salary is paid by the University of Geneva... Vincent Baujard, from the HON Foundation, Geneva, Switzerland developed the software for data collection. Thomas Eissenberg (Virginia Commonwealth University, USA), was a consultant for this study... | ***Acknowledgment: This study was partly funded by the Swiss Tobacco Prevention Fund (Swiss Federal Office of Public Health), grant 12.000189 to JFE. The Swiss Tobacco Prevention Fund had no role in the design or conduct of the study, interpretation of the data or decision to submit the paper for publication... JFE was reimbursed by Dekang, a manufacturer of e-cigarettes and e-liquids for traveling to London and to China, to visit e-cigarette factories, but he received no honoraria for these meetings. JFE's salary is paid by the University of Geneva... Vincent Baujard, from the HON Foundation, Geneva, Switzerland developed the software for data collection. Thomas Eissenberg (Virginia Commonwealth University, USA), was a consultant for this study... | ||
='''Heated Tobacco Product (HTP)'''= | |||
===2022 [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9424205/ An abuse liability assessment of the glo tobacco heating product in comparison to combustible cigarettes and nicotine replacement therapy]=== | |||
*"These findings suggest that the abuse liability of the THPs lies between that of subjects usual brand cigarettes and the NRT." | |||
**Citation: Hardie G, Gale N, McEwan M, Oscar SM, Ziviani L, Proctor CJ, Murphy J. An abuse liability assessment of the glo tobacco heating product in comparison to combustible cigarettes and nicotine replacement therapy. Sci Rep. 2022 Aug 29;12(1):14701. doi: 10.1038/s41598-022-19167-8. Erratum in: Sci Rep. 2023 Jun 27;13(1):10441. doi: 10.1038/s41598-023-37432-2. PMID: 36038580; PMCID: PMC9424205. | |||
***Acknowledgment: The study was funded in full by British American Tobacco (Investments) Limited (BAT). GH, NG, and MMcE are current employees of BAT. JM was an employee of BAT at the time of the study conduct and is currently an employee of R. J. Reynolds Tobacco Company, a subsidiary of BAT. CJP was an employee of BAT at the time of study conduct and is currently contracted to BAT to provide consultancy on tobacco product science and regulation. SM and LZ are employees of CRC, the clinic who performed the trial. | |||
='''Nicotine Pouches'''= | ='''Nicotine Pouches'''= | ||
| Line 156: | Line 218: | ||
***Acknowledgment: Grants: U54CA287392/DA/NIDA NIH HHS/United States, CA/NCI NIH HHS/United States | ***Acknowledgment: Grants: U54CA287392/DA/NIDA NIH HHS/United States, CA/NCI NIH HHS/United States | ||
=== | ='''Heated Tobacco Product (HTP)'''= | ||
*" | |||
**Citation: | ===2022 [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9424205/ An abuse liability assessment of the glo tobacco heating product in comparison to combustible cigarettes and nicotine replacement therapy]=== | ||
***Acknowledgment: | *"These findings suggest that the abuse liability of the THPs lies between that of subjects usual brand cigarettes and the NRT." | ||
**Citation: Hardie G, Gale N, McEwan M, Oscar SM, Ziviani L, Proctor CJ, Murphy J. An abuse liability assessment of the glo tobacco heating product in comparison to combustible cigarettes and nicotine replacement therapy. Sci Rep. 2022 Aug 29;12(1):14701. doi: 10.1038/s41598-022-19167-8. Erratum in: Sci Rep. 2023 Jun 27;13(1):10441. doi: 10.1038/s41598-023-37432-2. PMID: 36038580; PMCID: PMC9424205. | |||
***Acknowledgment: The study was funded in full by British American Tobacco (Investments) Limited (BAT). GH, NG, and MMcE are current employees of BAT. JM was an employee of BAT at the time of the study conduct and is currently an employee of R. J. Reynolds Tobacco Company, a subsidiary of BAT. CJP was an employee of BAT at the time of study conduct and is currently contracted to BAT to provide consultancy on tobacco product science and regulation. SM and LZ are employees of CRC, the clinic who performed the trial. | |||
='''Nicotine Replacement Therapy (NRT)'''= | ='''Nicotine Replacement Therapy (NRT)'''= | ||
| Line 167: | Line 231: | ||
**Citation: Campbell C, Jin T, Round EK, Schmidt E, Nelson P, Baxter S. Part one: abuse liability of Vuse Solo (G2) electronic nicotine delivery system relative to combustible cigarettes and nicotine gum. Sci Rep. 2022 Dec 21;12(1):22080. doi: 10.1038/s41598-022-26417-2. PMID: 36543869; PMCID: PMC9772348. | **Citation: Campbell C, Jin T, Round EK, Schmidt E, Nelson P, Baxter S. Part one: abuse liability of Vuse Solo (G2) electronic nicotine delivery system relative to combustible cigarettes and nicotine gum. Sci Rep. 2022 Dec 21;12(1):22080. doi: 10.1038/s41598-022-26417-2. PMID: 36543869; PMCID: PMC9772348. | ||
***Acknowledgment: C.C., T.J., E.S., E.R., and S.B. are full-time employees of RAI Services Company, and P.N. is a former full-time employee of RAI Services Company. RAI Services Company is a wholly owned subsidiary of Reynolds American Inc., which is an indirect, wholly owned subsidiary of British American Tobacco plc. | ***Acknowledgment: C.C., T.J., E.S., E.R., and S.B. are full-time employees of RAI Services Company, and P.N. is a former full-time employee of RAI Services Company. RAI Services Company is a wholly owned subsidiary of Reynolds American Inc., which is an indirect, wholly owned subsidiary of British American Tobacco plc. | ||
===2022 [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9424205/ An abuse liability assessment of the glo tobacco heating product in comparison to combustible cigarettes and nicotine replacement therapy]=== | |||
*"These findings suggest that the abuse liability of the THPs lies between that of subjects usual brand cigarettes and the NRT." | |||
**Citation: Hardie G, Gale N, McEwan M, Oscar SM, Ziviani L, Proctor CJ, Murphy J. An abuse liability assessment of the glo tobacco heating product in comparison to combustible cigarettes and nicotine replacement therapy. Sci Rep. 2022 Aug 29;12(1):14701. doi: 10.1038/s41598-022-19167-8. Erratum in: Sci Rep. 2023 Jun 27;13(1):10441. doi: 10.1038/s41598-023-37432-2. PMID: 36038580; PMCID: PMC9424205. | |||
***Acknowledgment: The study was funded in full by British American Tobacco (Investments) Limited (BAT). GH, NG, and MMcE are current employees of BAT. JM was an employee of BAT at the time of the study conduct and is currently an employee of R. J. Reynolds Tobacco Company, a subsidiary of BAT. CJP was an employee of BAT at the time of study conduct and is currently contracted to BAT to provide consultancy on tobacco product science and regulation. SM and LZ are employees of CRC, the clinic who performed the trial. | |||
===2020 [https://pubmed.ncbi.nlm.nih.gov/33176942/ Abuse liability assessment of the JUUL system in four flavors relative to combustible cigarette, nicotine gum and a comparator electronic nicotine delivery system among adult smokers]=== | ===2020 [https://pubmed.ncbi.nlm.nih.gov/33176942/ Abuse liability assessment of the JUUL system in four flavors relative to combustible cigarette, nicotine gum and a comparator electronic nicotine delivery system among adult smokers]=== | ||
| Line 177: | Line 246: | ||
**Citation: Goldenson NI, Buchhalter AR, Augustson EM, Rubinstein ML, Van Hoof D, Henningfield JE. Abuse liability assessment of the JUUL system in two nicotine concentrations compared to combustible cigarette, nicotine gum and comparator electronic nicotine delivery system. Drug Alcohol Depend. 2020 Dec 1;217:108441. doi: 10.1016/j.drugalcdep.2020.108441. Epub 2020 Nov 24. PMID: 33250386. | **Citation: Goldenson NI, Buchhalter AR, Augustson EM, Rubinstein ML, Van Hoof D, Henningfield JE. Abuse liability assessment of the JUUL system in two nicotine concentrations compared to combustible cigarette, nicotine gum and comparator electronic nicotine delivery system. Drug Alcohol Depend. 2020 Dec 1;217:108441. doi: 10.1016/j.drugalcdep.2020.108441. Epub 2020 Nov 24. PMID: 33250386. | ||
***Acknowledgment: The study was funded by Juul Labs, Inc. NIG, EMA, DVH and MLR are full-time employees of Juul Labs, Inc., JEH and ARB are full-time employees of PinneyAssociates, Inc. PinneyAssociates provides consulting services on tobacco harm reduction on an exclusive basis to Juul Labs, Inc. Within the last two years, PinneyAssociates has consulted for British American Tobacco and Reynolds American Inc and subsidiaries on tobacco harm reduction. JEH co-holds a patent for a novel nicotine medication that has not been developed or commercialized. There are no other interests declared by authors. | ***Acknowledgment: The study was funded by Juul Labs, Inc. NIG, EMA, DVH and MLR are full-time employees of Juul Labs, Inc., JEH and ARB are full-time employees of PinneyAssociates, Inc. PinneyAssociates provides consulting services on tobacco harm reduction on an exclusive basis to Juul Labs, Inc. Within the last two years, PinneyAssociates has consulted for British American Tobacco and Reynolds American Inc and subsidiaries on tobacco harm reduction. JEH co-holds a patent for a novel nicotine medication that has not been developed or commercialized. There are no other interests declared by authors. | ||
===2019 [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6754311/ Abuse liability assessment of an electronic cigarette in combustible cigarette smokers]=== | |||
*"Collectively, the results of this study demonstrated that the ECIG device and liquids examined had moderate levels of abuse liability: on average lower than combustible cigarettes, but higher than an FDA-approved nicotine replacement therapy (i.e., nicotine inhaler)." | |||
**Citation: Maloney SF, Breland A, Soule EK, Hiler M, Ramôa C, Lipato T, Eissenberg T. Abuse liability assessment of an electronic cigarette in combustible cigarette smokers. Exp Clin Psychopharmacol. 2019 Oct;27(5):443-454. doi: 10.1037/pha0000261. Epub 2019 Feb 18. PMID: 30777773; PMCID: PMC6754311. | |||
***Acknowledgment: Funding; This study was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number P50DA036105 and U54DA036105 and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration. COI; Dr. Eissenberg is a paid consultant in litigation against the tobacco industry and is named on a patent for a device that measures the puffing behavior of ECIG users. | |||
===2018 [https://pubmed.ncbi.nlm.nih.gov/29725702/ Assessment of the abuse liability of three menthol Vuse Solo electronic cigarettes relative to combustible cigarettes and nicotine gum]=== | ===2018 [https://pubmed.ncbi.nlm.nih.gov/29725702/ Assessment of the abuse liability of three menthol Vuse Solo electronic cigarettes relative to combustible cigarettes and nicotine gum]=== | ||
| Line 183: | Line 257: | ||
**Citation: Stiles MF, Campbell LR, Jin T, Graff DW, Fant RV, Henningfield JE. Assessment of the abuse liability of three menthol Vuse Solo electronic cigarettes relative to combustible cigarettes and nicotine gum. Psychopharmacology (Berl). 2018 Jul;235(7):2077-2086. doi: 10.1007/s00213-018-4904-x. Epub 2018 May 3. PMID: 29725702; PMCID: PMC6015619. | **Citation: Stiles MF, Campbell LR, Jin T, Graff DW, Fant RV, Henningfield JE. Assessment of the abuse liability of three menthol Vuse Solo electronic cigarettes relative to combustible cigarettes and nicotine gum. Psychopharmacology (Berl). 2018 Jul;235(7):2077-2086. doi: 10.1007/s00213-018-4904-x. Epub 2018 May 3. PMID: 29725702; PMCID: PMC6015619. | ||
***Acknowledgement: ClinicalTrials.gov identifier: NCT02664012. MF Stiles, LR Campbell, and T Jin are full-time employees of RAI Services Company. RAI Services Company is a wholly owned subsidiary of Reynolds American Inc., which is a wholly owned subsidiary of British American Tobacco plc. DW Graff is a full-time employee of Celerion and provided the original draft of the manuscript. RV Fant and JE Henningfield are full-time employees of PinneyAssociates, which provides consulting services on smoking cessation and tobacco harm minimization (including nicotine replacement therapy and electronic vapor products) to Niconovum, USA, Inc., RJ Reynolds Vapor Company, and RAI Services Company (all subsidiaries of Reynolds American Inc.). JE Henningfield also owns an interest in intellectual property for a novel nicotine medication. Through PinneyAssociates, Fant and Henningfield provide consulting services to pharmaceutical companies on abuse potential assessment, and the regulation of substances with a potential for abuse. | ***Acknowledgement: ClinicalTrials.gov identifier: NCT02664012. MF Stiles, LR Campbell, and T Jin are full-time employees of RAI Services Company. RAI Services Company is a wholly owned subsidiary of Reynolds American Inc., which is a wholly owned subsidiary of British American Tobacco plc. DW Graff is a full-time employee of Celerion and provided the original draft of the manuscript. RV Fant and JE Henningfield are full-time employees of PinneyAssociates, which provides consulting services on smoking cessation and tobacco harm minimization (including nicotine replacement therapy and electronic vapor products) to Niconovum, USA, Inc., RJ Reynolds Vapor Company, and RAI Services Company (all subsidiaries of Reynolds American Inc.). JE Henningfield also owns an interest in intellectual property for a novel nicotine medication. Through PinneyAssociates, Fant and Henningfield provide consulting services to pharmaceutical companies on abuse potential assessment, and the regulation of substances with a potential for abuse. | ||
===2017 [https://pubmed.ncbi.nlm.nih.gov/28634710/ Pharmacodynamic and pharmacokinetic assessment of electronic cigarettes, combustible cigarettes, and nicotine gum: implications for abuse liability]=== | |||
*"In summary, this study is the most robust assessment of the abuse liability of ECs published to date and uses approaches similar to those found in classic abuse liability studies of pharmaceutical products, including multiple instruments to measure the subjective effects of product use, as well as nicotine uptake. Under the set of study conditions described herein, use of the three Vuse Solo ECs tended to result in subjective measures responses and nicotine uptake that were between those measured with use of combustible cigarettes and nicotine gum. In general, the results are consistent with the conclusions of others that the abuse liability of ECs as a category is less than that of combustible cigarettes but greater than for nicotine gum, and likely other nicotine replacement products' | |||
**Citation: Stiles MF, Campbell LR, Graff DW, Jones BA, Fant RV, Henningfield JE. Pharmacodynamic and pharmacokinetic assessment of electronic cigarettes, combustible cigarettes, and nicotine gum: implications for abuse liability. Psychopharmacology (Berl). 2017 Sep;234(17):2643-2655. doi: 10.1007/s00213-017-4665-y. Epub 2017 Jun 20. PMID: 28634710; PMCID: PMC5548902. | |||
***Acknowledgment: Funding; This study was funded by RJ Reynolds Vapor Company through its affiliate RJ Reynolds Tobacco Company. COI; MF Stiles, LR Campbell, and BA Jones are full-time employees of RAI Services Company, which provides support across the Reynolds American Inc. operating companies. DW Graff is a full-time employee of Celerion and provided the original draft of this manuscript. RV Fant and JE Henningfield are full-time employees of PinneyAssociates, which provides consulting services on tobacco harm minimization (including nicotine replacement therapy and digital vapor products) to Niconovum USA, RJ Reynolds Vapor Company, and RAI Services Company (all subsidiaries of Reynolds American Inc.) In the past 3 years, PinneyAssociates has consulted to GlaxoSmithKline Consumer Healthcare on smoking cessation and NJOY on electronic cigarettes. JE Henningfield also owns an interest in intellectual property for a novel nicotine medication, an option for which has been sold to Niconovum USA. Through PinneyAssociates, Fant and Henningfield also provide consulting services to pharmaceutical companies on abuse potential assessment and the regulation of substances with a potential for abuse. | |||
===2015 [https://pubmed.ncbi.nlm.nih.gov/25561385/ Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes]=== | ===2015 [https://pubmed.ncbi.nlm.nih.gov/25561385/ Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes]=== | ||
* | *Conclusion: "Conclusions: Some e-cigarette users were dependent on nicotine-containing e-cigarettes, but these products were less addictive than tobacco cigarettes." | ||
**Citation: Etter JF, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015 Feb 1;147:68-75. doi: 10.1016/j.drugalcdep.2014.12.007. Epub 2014 Dec 18. PMID: 25561385; PMCID: PMC4920051. | |||
***This study was partly funded by the Swiss Tobacco Prevention Fund (Swiss Federal Office of Public Health), grant 12.000189 to JFE. The Swiss Tobacco Prevention Fund had no role in the design or conduct of the study, interpretation of the data or decision to submit the paper for publication. TE is supported by the National Institute on Drug Abuse of the U.S. National Institutes of Health under Award Number P50DA036105 and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration. | |||
===2013 [https://www.federalregister.gov/documents/2013/04/02/2013-07528/modifications-to-labeling-of-nicotine-replacement-therapy-products-for-over-the-counter-human-use Modifications To Labeling of Nicotine Replacement Therapy Products for Over-the-Counter Human Use]=== | ===2013 [https://www.federalregister.gov/documents/2013/04/02/2013-07528/modifications-to-labeling-of-nicotine-replacement-therapy-products-for-over-the-counter-human-use Modifications To Labeling of Nicotine Replacement Therapy Products for Over-the-Counter Human Use]=== | ||
| Line 203: | Line 284: | ||
**Citation: Houtsmuller EJ, Fant RV, Eissenberg TE, Henningfield JE, Stitzer ML. Flavor improvement does not increase abuse liability of nicotine chewing gum. Pharmacol Biochem Behav. 2002 Jun;72(3):559-68. doi: 10.1016/s0091-3057(02)00723-2. PMID: 12175452. | **Citation: Houtsmuller EJ, Fant RV, Eissenberg TE, Henningfield JE, Stitzer ML. Flavor improvement does not increase abuse liability of nicotine chewing gum. Pharmacol Biochem Behav. 2002 Jun;72(3):559-68. doi: 10.1016/s0091-3057(02)00723-2. PMID: 12175452. | ||
***Acknowledgement: This study was supported by SmithKline Beecham Consumer Healthcare. | ***Acknowledgement: This study was supported by SmithKline Beecham Consumer Healthcare. | ||
===2000 [https://pubmed.ncbi.nlm.nih.gov/10823399/ A comparison of the abuse liability and dependence potential of nicotine patch, gum, spray and inhaler]=== | |||
*"We conclude that abuse liability from all four NRT products was low. Subjective dependence was moderate and did not differ across products. Behavioural dependence was modest and was positively related to rate of nicotine delivery." | |||
**Citation: West R, Hajek P, Foulds J, Nilsson F, May S, Meadows A. A comparison of the abuse liability and dependence potential of nicotine patch, gum, spray and inhaler. Psychopharmacology (Berl). 2000 Apr;149(3):198-202. doi: 10.1007/s002130000382. PMID: 10823399. | |||
***Acknowledgment: This study was funded by Pharmacia and Upjohn, Sweden. | |||
===1997 [https://pubmed.ncbi.nlm.nih.gov/9160851/ Nicotine nasal spray and vapor inhaler: abuse liability assessment]=== | |||
*"Overall, results are consistent with the conclusion that the nicotine nasal spray and vapor inhaler are of substantially lower abuse liability than cigarettes in experienced cigarette smokers receiving initial exposure to these products." | |||
**Citation: Schuh KJ, Schuh LM, Henningfield JE, Stitzer ML. Nicotine nasal spray and vapor inhaler: abuse liability assessment. Psychopharmacology (Berl). 1997 Apr;130(4):352-61. doi: 10.1007/s002130050250. PMID: 9160851. | |||
***Acknowledgment: This work was supported by USPHS research grant DA03893 and training grant T32 DA07209 from the National Institute on Drug Abuse and conducted in collaboration with the NIDA Intramural Research Program Addiction Research Center. The authors thank Pharmacia Upjohn who kindly donated pharmaceutical supplies and conducted blood assays for this study. | |||
='''Novel Oral Products (Chewable)'''= | |||
===2021 [https://pubmed.ncbi.nlm.nih.gov/33502815/ Characterization of the Abuse Potential in Adult Smokers of a Novel Oral Tobacco Product Relative to Combustible Cigarettes and Nicotine Polacrilex Gum]=== | |||
*"The test products, under the conditions of this study, carry lower abuse potential than own-brand cigarettes and similar to nicotine polacrilex gum." | |||
**Citation: Liu J, Wang J, Vansickel A, Edmiston J, Graff D, Sarkar M. Characterization of the Abuse Potential in Adult Smokers of a Novel Oral Tobacco Product Relative to Combustible Cigarettes and Nicotine Polacrilex Gum. Clin Pharmacol Drug Dev. 2021 Mar;10(3):241-250. doi: 10.1002/cpdd.909. Epub 2021 Jan 27. PMID: 33502815; PMCID: PMC7986766. | |||
***Acknowledgment: J.L., J.W., A.V., J.E., and M.S. are employees of Altria Client Services LLC. D.G. was an employee of Celerion, Inc., who was contracted by Altria Client Services LLC to perform the study and analyze the study data. | |||
='''VLNC - Very Low Nicotine Cigarettes'''= | ='''VLNC - Very Low Nicotine Cigarettes'''= | ||
| Line 244: | Line 342: | ||
='''Suggestions to add to this page'''= | ='''Suggestions to add to this page'''= | ||
===2024: [https://osf.io/rf74g Chapter 26 Tobacco smoking addiction and nicotine dependence]=== | |||
*Sharon Cox | |||
===2016 [https://www.frontiersin.org/journals/psychiatry/articles/10.3389/fpsyt.2016.00126/full Belief about Nicotine Modulates Subjective Craving and Insula Activity in Deprived Smokers]=== | |||
Latest revision as of 10:02, 1 October 2024

Addiction: "A mental disposition towards repeated episodes of abnormally high levels of motivation to engage in a behaviour, acquired as a result of engaging in the behaviour, where the behaviour results in risk or occurrence of serious net harm."
Dependence: "A bodily disposition which is realised as impaired functioning following reduction or termination of use of a psychoactive substance. This is distinguished from addiction. The two are often correlated but distinct. Dependence is a disposition to experience impaired functioning, which includes withdrawal symptoms, while addiction is a distinction to experience powerful motivation. The motivation can, but need not be caused by impaired functioning."
Abuse Liability: The potential to develop a dependence or addiction to a substance.
Recommended Podcast: The Studies Show Episode 40: Addiction
Recommended Video: Tobacco Addiction & Nicotine Dependency
Background Information
- The Penn State Nicotine Dependence Index was developed by Dr. Jonathan Foulds in 2011. This 10-item scale (with scores ranging from 0 to 20) was developed to measure nicotine dependence across all nicotine product types, and an adapted version was the first dependence measure designed to evaluate electronic cigarette dependence.
- In the 2009 law giving FDA regulation over tobacco products, FDA is now required to evaluate new tobacco products including MRTP/PREPs to determine their risk for abuse and toxicity at the population level. This article describes the traditional tools and methods of ALA that can be used to evaluate new tobacco and nicotine products including MRTP/PREPs. Such ALA data could contribute to the scientific foundation on which future public policy decisions are based.
- The aim of this study was to develop a new, self-administered measure of cigarette dependence.
- The Fagerström Test for Nicotine Dependence is a standard instrument for assessing the intensity of physical addiction to nicotine. The test was designed to provide an ordinal measure of nicotine dependence related to cigarette smoking. It contains six items that evaluate the quantity of cigarette consumption, the compulsion to use, and dependence.
Smoking - Combustible Tobacco
2012 Determinants of Tobacco Use and Renaming the FTND to the Fagerström Test for Cigarette Dependence
- More recently, it has been found that, although nicotine is the most important addictive component of tobacco smoke, it is probably not the only substance involved in the development of tobacco dependence. In light of what is now known about what determines cigarette smoking, it seems timely to propose a renaming of the Fagerstrom Test for Nicotine Dependence (FTND) to the Fagerstrom Test for Cigarette Dependence (FTCD).
- See Also: 2013: Dependence on tobacco and nicotine
- PDF Version
- Citation: Karl Fagerström, Ph.D., Determinants of Tobacco Use and Renaming the FTND to the Fagerström Test for Cigarette Dependence, Nicotine & Tobacco Research, Volume 14, Issue 1, January 2012, Pages 75–78, doi: 10.1093/ntr/ntr137
- No COI or funding to declare.
- Citation: Karl Fagerström, Ph.D., Determinants of Tobacco Use and Renaming the FTND to the Fagerström Test for Cigarette Dependence, Nicotine & Tobacco Research, Volume 14, Issue 1, January 2012, Pages 75–78, doi: 10.1093/ntr/ntr137
2010 Nicotine Addiction
- Conslusions: "Nicotine sustains tobacco addiction, a major cause of disability and premature death, by acting on nicotinic cholinergic receptors in the brain to trigger the release of dopamine and other neurotransmitters. Release of dopamine, glutamate, and GABA is particularly important in the development of nicotine dependence, and CRF may play a key role in withdrawal. Neuroadaptation and tolerance involve changes in nicotinic receptors and neural plasticity. Nicotine addiction occurs when smokers come to rely on smoking to modulate mood and arousal, relieve withdrawal symptoms, or both. Light or occasional smokers smoke mainly for positive reinforcement in specific situations.
- Genetic studies indicate that nicotinic receptor subtypes and the genes involved in neuroplasticity and learning play a part in the development of dependence.
- People with psychiatric or substance-abuse disorders, who account for a large proportion of current smokers, have an increased susceptibility to tobacco addiction.
- Nicotine is metabolized primarily by the enzyme CYP2A6, and variation in the rate of nicotine metabolism contributes to differences in vulnerability to tobacco dependence and the response to smoking-cessation treatment. An increased understanding of the mechanisms of nicotine addiction has led to the development of novel medications (e.g., varenicline) that act on specific nicotinic receptor subtypes. The development of other drugs that act on nicotinic receptors and other mediators of nicotine addiction is likely to further enhance the effectiveness of smoking-cessation pharmacotherapy.
- Citation: Benowitz NL. Nicotine addiction. N Engl J Med. 2010 Jun 17;362(24):2295-303. doi: 10.1056/NEJMra0809890. PMID: 20554984; PMCID: PMC2928221.
- Acknowledgment: Supported by grants from the Flight Attendants Medical Research Institute and the National Institute on Drug Abuse (U.S. Public Health Service grants DA02277 and DA20830).
- Citation: Benowitz NL. Nicotine addiction. N Engl J Med. 2010 Jun 17;362(24):2295-303. doi: 10.1056/NEJMra0809890. PMID: 20554984; PMCID: PMC2928221.
ENDS/E-Cigarettes/NVP/Vaping vs Smoking Tobacco
- ENDS = Electronic Nicotine Delivery System
- NVP = Nicotine Vapor Product
- Conclusion: "In this longitudinal study of US adults who smoked cigarettes and switched completely or partially to JUUL, dependence on JUUL was lower than baseline dependence on cigarettes after a year of JUUL use among participants who smoked every day at baseline. Observed increases in JUUL dependence over 12 months of JUUL use were statistically significant but small in magnitude—lower than the estimated minimal important difference—suggesting that dependence on JUUL did not meaningfully increase over a 1-year period. Additional longitudinal data over longer periods of time is needed to more completely address trajectories of dependence on ENDS, including JUUL."
- Citation: Shiffman S, Goldenson NI. Changes in dependence over one year among US adults who smoke cigarettes and switched completely or partially to use of the JUUL-brand electronic nicotine delivery system. Drug Alcohol Depend Rep. 2023 Jan 26;6:100137. doi: 10.1016/j.dadr.2023.100137. PMID: 36994368; PMCID: PMC10040328.
- Acknowledgment: The study was funded by Juul Labs, Inc. SS is a senior advisor to PinneyAssociates, Inc, through which he provides consulting services on tobacco harm reduction on an exclusive basis to Juul Labs, Inc. NIG is a full-time employee of Juul Labs, Inc. The authors would like to acknowledge the Centre for Substance Use Research (CSUR), an independent research consultancy, for designing the ADJUSST study and collecting the data used in this manuscript.
- Citation: Shiffman S, Goldenson NI. Changes in dependence over one year among US adults who smoke cigarettes and switched completely or partially to use of the JUUL-brand electronic nicotine delivery system. Drug Alcohol Depend Rep. 2023 Jan 26;6:100137. doi: 10.1016/j.dadr.2023.100137. PMID: 36994368; PMCID: PMC10040328.
- "Results: In the sample, 27.6% were cigarette-only smokers, 24.8% were CIG-ECIG, 27.6% were CIG-CAN, and 20.0% were CIG-ECIG-CAN co-users. Significant differences were observed in sociodemographic and tobacco/other substance use characteristics by co-use status. E-cigarette co-users had low e-cigarette dependence, but moderate FTND scores. In adjusted analyses, only CIG-ECIG co-use was associated with higher FTND scores compared to cigarette-only smoking. However, CIG-ECIG and CIG-ECIG-CAN co-use were associated with higher FTND scores compared to CIG-CAN co-use."
- Citation: Jones DM, Guy MC, Fairman BJ, Soule E, Eissenberg T, Fagan P. Nicotine Dependence among Current Cigarette Smokers Who Use E-Cigarettes and Cannabis. Subst Use Misuse. 2023;58(5):618-628. doi: 10.1080/10826084.2023.2177961. Epub 2023 Feb 27. PMID: 36852436; PMCID: PMC10249428.
- Acknowledgment: Funding; K01 DA055088/DA/NIDA NIH HHS/United States and U54 DA036105/DA/NIDA NIH HHS/United States. COI; Thomas Eissenberg is a paid consultant in litigation against the tobacco industry and also the electronic cigarette industry and is named on one patent for a device that measures the puffing behavior of electronic cigarette users, on another patent application for a smartphone app that determines electronic cigarette device and liquid characteristics, and a third patent application for a smoking cessation intervention. Eric Soule is named on a patent application for a smartphone app that determines electronic cigarette device and liquid characteristics. The other authors declare they have no conflicts of interest.
- Citation: Jones DM, Guy MC, Fairman BJ, Soule E, Eissenberg T, Fagan P. Nicotine Dependence among Current Cigarette Smokers Who Use E-Cigarettes and Cannabis. Subst Use Misuse. 2023;58(5):618-628. doi: 10.1080/10826084.2023.2177961. Epub 2023 Feb 27. PMID: 36852436; PMCID: PMC10249428.
- "These data reinforce previous research and provide the scientific evidence to support regulatory decisions demonstrating that Vuse Solo has an AL profile lower than that of combustible cigarettes but higher than that of nicotine gum and, therefore, may be a suitable replacement for cigarette smoking for some adult smokers."
- Citation: Campbell C, Jin T, Round EK, Schmidt E, Nelson P, Baxter S. Part one: abuse liability of Vuse Solo (G2) electronic nicotine delivery system relative to combustible cigarettes and nicotine gum. Sci Rep. 2022 Dec 21;12(1):22080. doi: 10.1038/s41598-022-26417-2. PMID: 36543869; PMCID: PMC9772348.
- Acknowledgment: C.C., T.J., E.S., E.R., and S.B. are full-time employees of RAI Services Company, and P.N. is a former full-time employee of RAI Services Company. RAI Services Company is a wholly owned subsidiary of Reynolds American Inc., which is an indirect, wholly owned subsidiary of British American Tobacco plc.
- Citation: Campbell C, Jin T, Round EK, Schmidt E, Nelson P, Baxter S. Part one: abuse liability of Vuse Solo (G2) electronic nicotine delivery system relative to combustible cigarettes and nicotine gum. Sci Rep. 2022 Dec 21;12(1):22080. doi: 10.1038/s41598-022-26417-2. PMID: 36543869; PMCID: PMC9772348.
- "Conclusions: On average, JUUL users reported low to medium nicotine dependence on the PSECDI. JUUL user dependence may be more similar to e-cig user dependence than cigarette smoker dependence. These preliminary findings should be followed up in studies of larger samples of Juul users, collecting multiple measures of dependence, as well as biomarkers of nicotine intake (e.g. cotinine)."
- Citation: Yingst J, Foulds J, Hobkirk AL. Dependence and Use Characteristics of Adult JUUL Electronic Cigarette Users. Subst Use Misuse. 2021;56(1):61-66. doi: 10.1080/10826084.2020.1834582. Epub 2020 Oct 29. PMID: 33118854; PMCID: PMC7905831.
- Acknowledgment: Funding; This study was funded by internal funds provided by the Penn State College of Medicine Department of Psychiatry. ALH is supported by a career development award from the National Institute on Drug Abuse (K23 DA045081). JY and JF are supported by NIH grants (R01 DA048428, U01 DA045517). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. COI; JF has done paid consulting for pharmaceutical companies involved in producing smoking cessation medications, including GSK, Pfizer, Novartis, J&J, and Cypress Bioscience. The other authors have no disclosures to report related to this publication.
- Citation: Yingst J, Foulds J, Hobkirk AL. Dependence and Use Characteristics of Adult JUUL Electronic Cigarette Users. Subst Use Misuse. 2021;56(1):61-66. doi: 10.1080/10826084.2020.1834582. Epub 2020 Oct 29. PMID: 33118854; PMCID: PMC7905831.
- Among US high school students, increases in the prevalence of nicotine product use from 2012 to 2019 do not appear to have been accompanied by a similar increase in the population burden of nicotine dependence. This may be at least partly attributable to a shift in the most common product of choice from cigarettes (on which users are most dependent) to e-cigarettes (on which users are least dependent).
- Use of e-cigarettes increased dramatically, use of cigarettes declined, and use of combustible (non-cigarette) and smokeless tobacco was relatively stable. Whether the overall increase in product use has been mirrored by an increase in nicotine dependence was unclear.
- We found that different tobacco products were associated with differing levels of nicotine dependence, with cigarettes characterised by highest dependence and e-cigarettes in otherwise tobacco-naïve students by low dependence.
- The increase in population use of tobacco products between 2012 and 2019 (from 23.2% to 31.2%) was not accompanied by an equivalent increase in overall population burden of dependence (craving: 10.9% to 9.5%; wanting to use within 30min: 4.7% to 5.4%).
- PDF Version
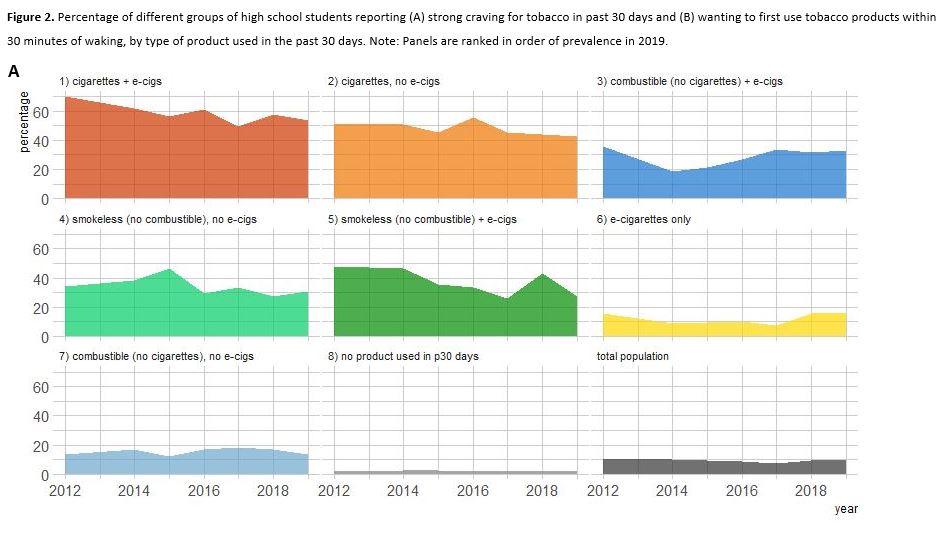
- Citation: Jackson, S. E., Brown, J., and Jarvis, M. J. (2021) Dependence on nicotine in US high school students in the context of changing patterns of tobacco product use. Addiction, 116: 1859– 1870. doi: 10.1111/add.15403
- Acknowledgement: Cancer Research UK (C1417/A22962) supported S.J. andJ.B.’s salaries.
- Citation: Jackson, S. E., Brown, J., and Jarvis, M. J. (2021) Dependence on nicotine in US high school students in the context of changing patterns of tobacco product use. Addiction, 116: 1859– 1870. doi: 10.1111/add.15403
- Conclusions: Dependence decreased as smokers transitioned from smoking to exclusive use of JUUL, similarly for users of both nicotine concentrations. Smokers who switch to JUUL may reduce their nicotine dependence.
- Citation: Shiffman S, Goldenson NI, Hatcher C, Augustson EM. Changes in Dependence as Smokers Switch from Cigarettes to JUUL in Two Nicotine Concentrations. Am J Health Behav. 2021 May 1;45(3):563-575. doi: 10.5993/AJHB.45.3.10. PMID: 33894800.
- Acknowledgement: NIG, CH, and EMA are full-time employees of Juul Labs Inc. Through Pinney Associates, SS provides consulting services on tobacco harm reduction on an exclusive basis to Juul Labs Inc. Within the last 2 years, PinneyAssociates has consulted for British American Tobacco and Reynolds American Inc and subsidiaries on tobacco harm reduction. Under contract to JUUL Labs Inc, the Centre for Substance Use Research, an independent research consultancy, designed the study and assessments and oversaw collection of data through Dacima, Inc. CSUR managed, cleaned, and summarized the data. CH performed the statistical analyses under the direction of SS and NG. All of the authors contributed to writing and review of the paper, and had access to the data. The sponsor approved the research plan and provided comment on a nearfinal draft of the paper. Funding for this study was provided by Juul Labs Inc.
- Citation: Shiffman S, Goldenson NI, Hatcher C, Augustson EM. Changes in Dependence as Smokers Switch from Cigarettes to JUUL in Two Nicotine Concentrations. Am J Health Behav. 2021 May 1;45(3):563-575. doi: 10.5993/AJHB.45.3.10. PMID: 33894800.
- Conclusions: Controlled use of JS among adult smokers resulted in nicotine delivery, product liking, and satisfaction that were less than that of combustible cigarettes but generally greater than nicotine gum. These results support the conclusion that JS has lower abuse liability than combustible cigarettes, higher abuse liability than nicotine gum, and may provide sufficient nicotine delivery and satisfying effects to support substitution for combustible cigarettes among adult smokers.
- Citation: Goldenson NI, Buchhalter AR, Augustson EM, Rubinstein ML, Henningfield JE. Abuse liability assessment of the JUUL system in four flavors relative to combustible cigarette, nicotine gum and a comparator electronic nicotine delivery system among adult smokers. Drug Alcohol Depend. 2020 Dec 1;217:108395. doi: 10.1016/j.drugalcdep.2020.108395. Epub 2020 Nov 4. PMID: 33176942.
- Acknowledgment: The study was funded by Juul Labs, Inc. NIG, EMA and MLR are full-time employees of Juul Labs, Inc., JEH and ARB are full-time employees of PinneyAssociates, Inc. PinneyAssociates provides consulting services on tobacco harm reduction on an exclusive basis to Juul Labs, Inc. Within the last two years, PinneyAssociates has consulted for British American Tobacco and Reynolds American Inc and subsidiaries on tobacco harm reduction. JEH co-holds a patent for a novel nicotine medication that has not been developed or commercialized. There are no other interests declared by authors.
- Citation: Goldenson NI, Buchhalter AR, Augustson EM, Rubinstein ML, Henningfield JE. Abuse liability assessment of the JUUL system in four flavors relative to combustible cigarette, nicotine gum and a comparator electronic nicotine delivery system among adult smokers. Drug Alcohol Depend. 2020 Dec 1;217:108395. doi: 10.1016/j.drugalcdep.2020.108395. Epub 2020 Nov 4. PMID: 33176942.
- Conclusions: These results suggest that the abuse liability of both 5.0 % and 3.0 % JS is: (1) substantially lower than UB cigarette; (2) somewhat lower than comparator ENDS; and (3) higher than nicotine gum. Additionally, the abuse liability of JS 5.0 % is somewhat higher than JS 3.0 %.
- Citation: Goldenson NI, Buchhalter AR, Augustson EM, Rubinstein ML, Van Hoof D, Henningfield JE. Abuse liability assessment of the JUUL system in two nicotine concentrations compared to combustible cigarette, nicotine gum and comparator electronic nicotine delivery system. Drug Alcohol Depend. 2020 Dec 1;217:108441. doi: 10.1016/j.drugalcdep.2020.108441. Epub 2020 Nov 24. PMID: 33250386.
- Acknowledgment: The study was funded by Juul Labs, Inc. NIG, EMA, DVH and MLR are full-time employees of Juul Labs, Inc., JEH and ARB are full-time employees of PinneyAssociates, Inc. PinneyAssociates provides consulting services on tobacco harm reduction on an exclusive basis to Juul Labs, Inc. Within the last two years, PinneyAssociates has consulted for British American Tobacco and Reynolds American Inc and subsidiaries on tobacco harm reduction. JEH co-holds a patent for a novel nicotine medication that has not been developed or commercialized. There are no other interests declared by authors.
- Citation: Goldenson NI, Buchhalter AR, Augustson EM, Rubinstein ML, Van Hoof D, Henningfield JE. Abuse liability assessment of the JUUL system in two nicotine concentrations compared to combustible cigarette, nicotine gum and comparator electronic nicotine delivery system. Drug Alcohol Depend. 2020 Dec 1;217:108441. doi: 10.1016/j.drugalcdep.2020.108441. Epub 2020 Nov 24. PMID: 33250386.
- Conclusion: "On average, JUUL users reported low to medium nicotine dependence on the PSECDI. JUUL user dependence may be more similar to e-cig user dependence than cigarette smoker dependence. These preliminary findings should be followed up in studies of larger samples of Juul users, collecting multiple measures of dependence, as well as biomarkers of nicotine intake (e.g. cotinine)."
- Citation: Yingst J, Foulds J, Hobkirk AL. Dependence and Use Characteristics of Adult JUUL Electronic Cigarette Users. Subst Use Misuse. 2021;56(1):61-66. doi: 10.1080/10826084.2020.1834582. Epub 2020 Oct 29. PMID: 33118854; PMCID: PMC7905831.
- Acknowledgment: This study was funded by internal funds provided by the Penn State College of Medicine Department of Psychiatry. ALH is supported by a career development award from the National Institute on Drug Abuse (K23 DA045081). JY and JF are supported by NIH grants (R01 DA048428, U01 DA045517). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. JF has done paid consulting for pharmaceutical companies involved in producing smoking cessation medications, including GSK, Pfizer, Novartis, J&J, and Cypress Bioscience. The other authors have no disclosures to report related to this publication.
- Citation: Yingst J, Foulds J, Hobkirk AL. Dependence and Use Characteristics of Adult JUUL Electronic Cigarette Users. Subst Use Misuse. 2021;56(1):61-66. doi: 10.1080/10826084.2020.1834582. Epub 2020 Oct 29. PMID: 33118854; PMCID: PMC7905831.
- "Conclusion: While there was no difference in CD between dual and cigarette only users, dual users' ED was lower than that for ENDS only users. ENDS appeared to produce less dependence than cigarettes among dual users. Given the high nicotine concentration ENDS products that entered the market after PATH Wave 3 data were collected, future research should examine ED among ENDS only and dual users."
- Citation: Kaplan B, Alrumaih F, Breland A, Eissenberg T, Cohen JE. A comparison of product dependence among cigarette only, ENDS only, and dual users: Findings from Wave 3 (2015-2016) of the PATH study. Drug Alcohol Depend. 2020 Dec 1;217:108347. doi: 10.1016/j.drugalcdep.2020.108347. Epub 2020 Oct 12. PMID: 33086157; PMCID: PMC7736550.
- Acknowledgment: This research was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number U54DA036105 and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the FDA. Dr. Eissenberg is a paid consultant in litigation against the tobacco industry and also the electronic cigarette industry and is named on one patent for a device that measures the puffing behavior of electronic cigarette users and on another patent for a smartphone app that determines electronic cigarette device and liquid characteristics. The other co-authors declare no conflict of interests.
- Citation: Kaplan B, Alrumaih F, Breland A, Eissenberg T, Cohen JE. A comparison of product dependence among cigarette only, ENDS only, and dual users: Findings from Wave 3 (2015-2016) of the PATH study. Drug Alcohol Depend. 2020 Dec 1;217:108347. doi: 10.1016/j.drugalcdep.2020.108347. Epub 2020 Oct 12. PMID: 33086157; PMCID: PMC7736550.
- Conclusion: "Use of e-cigarettes appears to be consistently associated with lower nicotine dependence than cigarette smoking."
- Citation: Shiffman S, Sembower MA. Dependence on e-cigarettes and cigarettes in a cross-sectional study of US adults. Addiction. 2020 Oct;115(10):1924-1931. doi: 10.1111/add.15060. Epub 2020 Apr 20. PMID: 32196810; PMCID: PMC7540348.
- Acknowledgment: The authors are grateful to Matthew Carpenter (Medical University of South Carolina), Michael Dunbar (RAND Corporation), and Jack Henningfield (Pinney Associates and Johns Hopkins University) for comments on an earlier draft. The authors also are grateful for the contributions of Mimi Kim, PhD., an employee of RAIS, who helped convey the PATH team's protocol for analyzing dependence measures. This research was supported by RAI Services Company, which had no role in its conception, analysis, writing, or decision to submit for publication. At the time the analysis was conducted, Pinney Associates, Inc., provided consulting services on tobacco harm minimization (including smokeless tobacco and vapor products) to R.J. Reynolds Vapor Company, and RAI Services Company, all of which are subsidiaries of Reynolds American Inc. Currently, Pinney Associates, and both authors, consult to JUUL Labs, Inc. regarding e-cigarettes and harm reduction. S.S. also owns an interest in intellectual property for a novel nicotine medication that has not been developed.
- Citation: Shiffman S, Sembower MA. Dependence on e-cigarettes and cigarettes in a cross-sectional study of US adults. Addiction. 2020 Oct;115(10):1924-1931. doi: 10.1111/add.15060. Epub 2020 Apr 20. PMID: 32196810; PMCID: PMC7540348.
- Conclusions: Controlled use of JS among adult smokers resulted in nicotine delivery, product liking, and satisfaction that were less than that of combustible cigarettes but generally greater than nicotine gum. These results support the conclusion that JS has lower abuse liability than combustible cigarettes, higher abuse liability than nicotine gum, and may provide sufficient nicotine delivery and satisfying effects to support substitution for combustible cigarettes among adult smokers.
- Citation: Goldenson NI, Buchhalter AR, Augustson EM, Rubinstein ML, Henningfield JE. Abuse liability assessment of the JUUL system in four flavors relative to combustible cigarette, nicotine gum and a comparator electronic nicotine delivery system among adult smokers. Drug Alcohol Depend. 2020 Dec 1;217:108395. doi: 10.1016/j.drugalcdep.2020.108395. Epub 2020 Nov 4. PMID: 33176942.
- Acknowledgment: The study was funded by Juul Labs, Inc. NIG, EMA and MLR are full-time employees of Juul Labs, Inc., JEH and ARB are full-time employees of PinneyAssociates, Inc. PinneyAssociates provides consulting services on tobacco harm reduction on an exclusive basis to Juul Labs, Inc. Within the last two years, PinneyAssociates has consulted for British American Tobacco and Reynolds American Inc and subsidiaries on tobacco harm reduction. JEH co-holds a patent for a novel nicotine medication that has not been developed or commercialized. There are no other interests declared by authors.
- Citation: Goldenson NI, Buchhalter AR, Augustson EM, Rubinstein ML, Henningfield JE. Abuse liability assessment of the JUUL system in four flavors relative to combustible cigarette, nicotine gum and a comparator electronic nicotine delivery system among adult smokers. Drug Alcohol Depend. 2020 Dec 1;217:108395. doi: 10.1016/j.drugalcdep.2020.108395. Epub 2020 Nov 4. PMID: 33176942.
- Conclusion: "Nearly 20% of dual users used ECs either without nicotine or without knowing if the product contained nicotine. The PSDI [Penn State Dependence Index] indicated greater dependence on smoking than vaping. Reasons for vaping were nearly equal between smoking reduction and enjoying flavors. Understanding patterns of dual use will inform future efforts to address nicotine dependence for AI communities with high prevalence of smoking."
- Citation: Rhoades DA, Comiford AL, Dvorak JD, Ding K, Hopkins M, Spicer P, Wagener TL, Doescher MP. Vaping patterns, nicotine dependence and reasons for vaping among American Indian dual users of cigarettes and electronic cigarettes. BMC Public Health. 2019 Sep 2;19(1):1211. doi: 10.1186/s12889-019-7523-5. PMID: 31477072; PMCID: PMC6721166.
- Acknowledgment: Grants from the National Cancer Institute of the National Institutes of Health (P20CA202921 to University of Oklahoma, and 5P20CA202923 to Cherokee Nation) supported this study. The funding body had no role in the design of the study and collection, analysis, interpretation of data, or writing the manuscript. Content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health or of Cherokee Nation. The authors declare that they have no competing interests.
- Citation: Rhoades DA, Comiford AL, Dvorak JD, Ding K, Hopkins M, Spicer P, Wagener TL, Doescher MP. Vaping patterns, nicotine dependence and reasons for vaping among American Indian dual users of cigarettes and electronic cigarettes. BMC Public Health. 2019 Sep 2;19(1):1211. doi: 10.1186/s12889-019-7523-5. PMID: 31477072; PMCID: PMC6721166.
- "Collectively, the results of this study demonstrated that the ECIG device and liquids examined had moderate levels of abuse liability: on average lower than combustible cigarettes, but higher than an FDA-approved nicotine replacement therapy (i.e., nicotine inhaler)."
- Citation: Maloney SF, Breland A, Soule EK, Hiler M, Ramôa C, Lipato T, Eissenberg T. Abuse liability assessment of an electronic cigarette in combustible cigarette smokers. Exp Clin Psychopharmacol. 2019 Oct;27(5):443-454. doi: 10.1037/pha0000261. Epub 2019 Feb 18. PMID: 30777773; PMCID: PMC6754311.
- Acknowledgment: Funding; This study was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number P50DA036105 and U54DA036105 and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration. COI; Dr. Eissenberg is a paid consultant in litigation against the tobacco industry and is named on a patent for a device that measures the puffing behavior of ECIG users.
- Citation: Maloney SF, Breland A, Soule EK, Hiler M, Ramôa C, Lipato T, Eissenberg T. Abuse liability assessment of an electronic cigarette in combustible cigarette smokers. Exp Clin Psychopharmacol. 2019 Oct;27(5):443-454. doi: 10.1037/pha0000261. Epub 2019 Feb 18. PMID: 30777773; PMCID: PMC6754311.
- These findings are concordant with our previous results and provide evidence that menthol Vuse Solo ECs have abuse liability that is lower than menthol cigarettes and potentially greater than that of nicotine gum.
- PDF Version
- Citation: Stiles MF, Campbell LR, Jin T, Graff DW, Fant RV, Henningfield JE. Assessment of the abuse liability of three menthol Vuse Solo electronic cigarettes relative to combustible cigarettes and nicotine gum. Psychopharmacology (Berl). 2018 Jul;235(7):2077-2086. doi: 10.1007/s00213-018-4904-x. Epub 2018 May 3. PMID: 29725702; PMCID: PMC6015619.
- Acknowledgement: ClinicalTrials.gov identifier: NCT02664012. MF Stiles, LR Campbell, and T Jin are full-time employees of RAI Services Company. RAI Services Company is a wholly owned subsidiary of Reynolds American Inc., which is a wholly owned subsidiary of British American Tobacco plc. DW Graff is a full-time employee of Celerion and provided the original draft of the manuscript. RV Fant and JE Henningfield are full-time employees of PinneyAssociates, which provides consulting services on smoking cessation and tobacco harm minimization (including nicotine replacement therapy and electronic vapor products) to Niconovum, USA, Inc., RJ Reynolds Vapor Company, and RAI Services Company (all subsidiaries of Reynolds American Inc.). JE Henningfield also owns an interest in intellectual property for a novel nicotine medication. Through PinneyAssociates, Fant and Henningfield provide consulting services to pharmaceutical companies on abuse potential assessment, and the regulation of substances with a potential for abuse.
- Citation: Stiles MF, Campbell LR, Jin T, Graff DW, Fant RV, Henningfield JE. Assessment of the abuse liability of three menthol Vuse Solo electronic cigarettes relative to combustible cigarettes and nicotine gum. Psychopharmacology (Berl). 2018 Jul;235(7):2077-2086. doi: 10.1007/s00213-018-4904-x. Epub 2018 May 3. PMID: 29725702; PMCID: PMC6015619.
- Conclusion: "First, we found that the large majority of vapers were ex-smokers who had either ceased or dramatically reduced their cigarette consumption. Second, there was a marked decrease in dependence among vapers compared to their retrospective prior cigarette dependence..."
- PDF of full study
- Citation: Browne M, Todd DG. Then and now: Consumption and dependence in e-cigarette users who formerly smoked cigarettes. Addict Behav. 2018 Jan;76:113-121. doi: 10.1016/j.addbeh.2017.07.034. Epub 2017 Jul 28. PMID: 28780356.
- Acknowledgement: Research was supported by block funding to the School of Health, Medical and Applied Sciences, Central Queensland University
- Citation: Browne M, Todd DG. Then and now: Consumption and dependence in e-cigarette users who formerly smoked cigarettes. Addict Behav. 2018 Jan;76:113-121. doi: 10.1016/j.addbeh.2017.07.034. Epub 2017 Jul 28. PMID: 28780356.
- "These findings offer possible support that e-cigarettes may act as a smoking reduction method among highly nicotine-dependent young adult cigarette smokers."
- Citation: Selya AS, Dierker L, Rose JS, Hedeker D, Mermelstein RJ. The Role of Nicotine Dependence in E-Cigarettes' Potential for Smoking Reduction. Nicotine Tob Res. 2018 Sep 4;20(10):1272-1277. doi: 10.1093/ntr/ntx160. PMID: 29065204; PMCID: PMC6121914.
- Acknowledgment: Grants and funding, P01 CA098262/CA/NCI NIH HHS/United States, P50 DA039838/DA/NIDA NIH HHS/United States
- Citation: Selya AS, Dierker L, Rose JS, Hedeker D, Mermelstein RJ. The Role of Nicotine Dependence in E-Cigarettes' Potential for Smoking Reduction. Nicotine Tob Res. 2018 Sep 4;20(10):1272-1277. doi: 10.1093/ntr/ntx160. PMID: 29065204; PMCID: PMC6121914.
- Conclusion: These results are consistent with previous studies, in finding that exclusive daily e-cigarette users are less dependent on their respective product than comparable cigarette smokers.
- Citation: Liu G, Wasserman E, Kong L, Foulds J. A comparison of nicotine dependence among exclusive E-cigarette and cigarette users in the PATH study. Prev Med. 2017 Nov;104:86-91. doi: 10.1016/j.ypmed.2017.04.001. Epub 2017 Apr 4. PMID: 28389330; PMCID: PMC5868349.
- Acknowledgement: This work was supported in part by the National Institute on Drug Abuse of the NIH and the Center for Tobacco Products of the FDA (P50-DA-036107) (Liu, Wasserman, Foulds) and by the National Center for Advancing Translational Sciences, NIH, through Grant UL1 TR000127 (Kong). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
- Citation: Liu G, Wasserman E, Kong L, Foulds J. A comparison of nicotine dependence among exclusive E-cigarette and cigarette users in the PATH study. Prev Med. 2017 Nov;104:86-91. doi: 10.1016/j.ypmed.2017.04.001. Epub 2017 Apr 4. PMID: 28389330; PMCID: PMC5868349.
- Conclusion: "Results showed that e-cigarette users scored lower than cigarette smokers in both FTND and all NDSS subscales. Our findings extend previous research on e-cigarette use and nicotine addiction and suggest that e-cigarette users are less dependent on nicotine than current tobacco cigarette smokers."
- Citation: González Roz A, Secades Villa R, Weidberg S. Evaluating nicotine dependence levels in e-cigarette users. Adicciones. 2017 Jan 11;29(2):136-138. English, Spanish. doi: 10.20882/adicciones.905. PMID: 28170058.
- Acknowledgment: Funding for this study was provided by the BBVA foundation (SV-14-FBBVA-1). This institution had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication. No conflicts reported.
- Citation: González Roz A, Secades Villa R, Weidberg S. Evaluating nicotine dependence levels in e-cigarette users. Adicciones. 2017 Jan 11;29(2):136-138. English, Spanish. doi: 10.20882/adicciones.905. PMID: 28170058.
- Conclusion: "Nicotine dependence is not a significant mechanism for e-cigarettes’ purported effect on heavier future conventional smoking among young adults. Nicotine dependence may be a mechanism for increases in e-cigarette use among heavier conventional smokers, consistent with e-cigarettes as a smoking reduction tool. Overall, conventional smoking and, to a lesser extent, its resulting nicotine dependence, are the strongest drivers or signals of later cigarette and e-cigarette use."
- Citation: Selya AS, Rose JS, Dierker L, Hedeker D, Mermelstein RJ. Evaluating the mutual pathways among electronic cigarette use, conventional smoking and nicotine dependence. Addiction. 2018 Feb;113(2):325-333. doi: 10.1111/add.14013. Epub 2017 Sep 25. PMID: 28841780; PMCID: PMC5760290.
- Acknowledgment: Grants and funding, L40 DA042431/DA/NIDA NIH HHS/United States, P01 CA098262/CA/NCI NIH HHS/United States, P30 DK092949/DK/NIDDK NIH HHS/United States, P50 DA039838/DA/NIDA NIH HHS/United States
- Citation: Selya AS, Rose JS, Dierker L, Hedeker D, Mermelstein RJ. Evaluating the mutual pathways among electronic cigarette use, conventional smoking and nicotine dependence. Addiction. 2018 Feb;113(2):325-333. doi: 10.1111/add.14013. Epub 2017 Sep 25. PMID: 28841780; PMCID: PMC5760290.
- Conclusion: "Our results were largely consistent with expectations. First, we found that the large majority of vapers were ex-smokers who had either ceased or dramatically reduced their cigarette consumption. Second, there was a marked decrease in dependence among vapers compared to their retrospective prior cigarette dependence. Finally, we also observed decoupling: a large attenuation of the relationship between dependence and consumption for vapers as compared to their retrospective prior smoking. We incorporated multiple measures of vaping consumption, which showed high variability with respect to a vapour volume/(negative) nicotine concentration continuum, with female and older vapers tending to vape at lower volumes combined with higher nicotine concentrations. However, the lack of reliability and unidimensionality of the FTND-V raise concerns about the adequacy of cigarette-analogous dependence measures for vaping, and whether ‘apples to apples’ comparisons with smoking are strictly valid. Finally, we observed no relationship between dependence or e-liquid volume consumption and duration of vaping. There was a tendency for those who have been vaping longer to employ increased nicotine concentration, but this was moderated by vapers' intentions to reduce their intake. Future research should focus on better measurement of consumption patterns and dependence indices for vaping, and employ these measures in prospective longitudinal designs."
- Citation: Browne M, Todd DG. Then and now: Consumption and dependence in e-cigarette users who formerly smoked cigarettes. Addict Behav. 2018 Jan;76:113-121. doi: 10.1016/j.addbeh.2017.07.034. Epub 2017 Jul 28. PMID: 28780356.
- Acknowledgement: Research was supported by block funding to the School of Health, Medical and Applied Sciences, Central Queensland University. No conflicts of interest to declare.
- Citation: Browne M, Todd DG. Then and now: Consumption and dependence in e-cigarette users who formerly smoked cigarettes. Addict Behav. 2018 Jan;76:113-121. doi: 10.1016/j.addbeh.2017.07.034. Epub 2017 Jul 28. PMID: 28780356.
- "In summary, this study is the most robust assessment of the abuse liability of ECs published to date and uses approaches similar to those found in classic abuse liability studies of pharmaceutical products, including multiple instruments to measure the subjective effects of product use, as well as nicotine uptake. Under the set of study conditions described herein, use of the three Vuse Solo ECs tended to result in subjective measures responses and nicotine uptake that were between those measured with use of combustible cigarettes and nicotine gum. In general, the results are consistent with the conclusions of others that the abuse liability of ECs as a category is less than that of combustible cigarettes but greater than for nicotine gum, and likely other nicotine replacement products'
- Citation: Stiles MF, Campbell LR, Graff DW, Jones BA, Fant RV, Henningfield JE. Pharmacodynamic and pharmacokinetic assessment of electronic cigarettes, combustible cigarettes, and nicotine gum: implications for abuse liability. Psychopharmacology (Berl). 2017 Sep;234(17):2643-2655. doi: 10.1007/s00213-017-4665-y. Epub 2017 Jun 20. PMID: 28634710; PMCID: PMC5548902.
- Acknowledgment: Funding; This study was funded by RJ Reynolds Vapor Company through its affiliate RJ Reynolds Tobacco Company. COI; MF Stiles, LR Campbell, and BA Jones are full-time employees of RAI Services Company, which provides support across the Reynolds American Inc. operating companies. DW Graff is a full-time employee of Celerion and provided the original draft of this manuscript. RV Fant and JE Henningfield are full-time employees of PinneyAssociates, which provides consulting services on tobacco harm minimization (including nicotine replacement therapy and digital vapor products) to Niconovum USA, RJ Reynolds Vapor Company, and RAI Services Company (all subsidiaries of Reynolds American Inc.) In the past 3 years, PinneyAssociates has consulted to GlaxoSmithKline Consumer Healthcare on smoking cessation and NJOY on electronic cigarettes. JE Henningfield also owns an interest in intellectual property for a novel nicotine medication, an option for which has been sold to Niconovum USA. Through PinneyAssociates, Fant and Henningfield also provide consulting services to pharmaceutical companies on abuse potential assessment and the regulation of substances with a potential for abuse.
- Citation: Stiles MF, Campbell LR, Graff DW, Jones BA, Fant RV, Henningfield JE. Pharmacodynamic and pharmacokinetic assessment of electronic cigarettes, combustible cigarettes, and nicotine gum: implications for abuse liability. Psychopharmacology (Berl). 2017 Sep;234(17):2643-2655. doi: 10.1007/s00213-017-4665-y. Epub 2017 Jun 20. PMID: 28634710; PMCID: PMC5548902.
- Conclusion: Current e-cigarette users reported being less dependent on e-cigarettes than they retrospectively reported having been dependent on cigarettes prior to switching. E-cig dependence appears to vary by product characteristics and liquid nicotine concentration, and it may increase over time.
- Citation: Foulds J, Veldheer S, Yingst J, Hrabovsky S, Wilson SJ, Nichols TT, Eissenberg T. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking E-cigarette users. Nicotine Tob Res. 2015 Feb;17(2):186-92. doi: 10.1093/ntr/ntu204. Epub 2014 Oct 19. PMID: 25332459; PMCID: PMC4838001.
- Acknowledgment: This work was supported by an internal grant from Penn State Social Science Research Institute and Cancer Institute (PI: SJW). JF, SV, JY, and SH are primarily funded by the National Institute on Drug Abuse of the National Institutes of Health and the Center for Tobacco Products of the US Food and Drug Administration (P50-DA-036107-01; and P50-DA-0361-05). TE is supported by the National Institute on Drug Abuse of the National Institutes of Health (P50-DA-0361-05) and the Center for Tobacco Products of the US Food and Drug Administration. JF has done paid consulting for pharmaceutical companies involved in producing smoking cessation medications, including GSK, Pfizer, Novartis, J&J, and Cypress Bioscience. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the US Food and Drug Administration.
- Citation: Foulds J, Veldheer S, Yingst J, Hrabovsky S, Wilson SJ, Nichols TT, Eissenberg T. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking E-cigarette users. Nicotine Tob Res. 2015 Feb;17(2):186-92. doi: 10.1093/ntr/ntu204. Epub 2014 Oct 19. PMID: 25332459; PMCID: PMC4838001.
- Conclusion: "Conclusions: Some e-cigarette users were dependent on nicotine-containing e-cigarettes, but these products were less addictive than tobacco cigarettes."
- Citation: Etter JF, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015 Feb 1;147:68-75. doi: 10.1016/j.drugalcdep.2014.12.007. Epub 2014 Dec 18. PMID: 25561385; PMCID: PMC4920051.
- This study was partly funded by the Swiss Tobacco Prevention Fund (Swiss Federal Office of Public Health), grant 12.000189 to JFE. The Swiss Tobacco Prevention Fund had no role in the design or conduct of the study, interpretation of the data or decision to submit the paper for publication. TE is supported by the National Institute on Drug Abuse of the U.S. National Institutes of Health under Award Number P50DA036105 and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
- Citation: Etter JF, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015 Feb 1;147:68-75. doi: 10.1016/j.drugalcdep.2014.12.007. Epub 2014 Dec 18. PMID: 25561385; PMCID: PMC4920051.
- Conclusion: Electronic cigarettes can deliver clinically significant amounts of nicotine and reduce cigarette abstinence symptoms and appear to have lower potential for abuse relative to traditional tobacco cigarettes, at least under certain laboratory conditions.
- Citation:Vansickel AR, Weaver MF, Eissenberg T. Clinical laboratory assessment of the abuse liability of an electronic cigarette. Addiction. 2012 Aug;107(8):1493-500. doi: 10.1111/j.1360-0443.2012.03791.x. Epub 2012 May 8. PMID: 22229871; PMCID: PMC3330136.
- Acknowledgment: This work was partially funded by the National Cancer Institute, National Institutes of Health RO1CA 120142 (awarded to Dr. Eissenberg). Dr. Vansickel was supported by National Institute on Drug Abuse, National Institutes of Health T32DA 007027. The authors have no conflicts of interest to declare.
- Citation:Vansickel AR, Weaver MF, Eissenberg T. Clinical laboratory assessment of the abuse liability of an electronic cigarette. Addiction. 2012 Aug;107(8):1493-500. doi: 10.1111/j.1360-0443.2012.03791.x. Epub 2012 May 8. PMID: 22229871; PMCID: PMC3330136.
ENDS/E-Cigarettes/NVP/Vaping - Not Compared with Smoking Tobacco
- Conclusion: "Most exclusive EC users in the study developed a moderate nicotine dependence level. The EC device type and nicotine concentration were significant drivers of nicotine dependence."
- Citation: Yahya L, Mandoura N, Harere R. Nicotine Dependency Levels Among Adult Electronic Cigarette Smokers in Jeddah, Saudi Arabia: An Analytical Cross-Sectional Study. Cureus. 2024 May 25;16(5):e61038. doi: 10.7759/cureus.61038. PMID: 38800771; PMCID: PMC11127123.
- Acknowledgment: The authors have declared that no competing interests exist. (No funding mentioned.)
- Citation: Yahya L, Mandoura N, Harere R. Nicotine Dependency Levels Among Adult Electronic Cigarette Smokers in Jeddah, Saudi Arabia: An Analytical Cross-Sectional Study. Cureus. 2024 May 25;16(5):e61038. doi: 10.7759/cureus.61038. PMID: 38800771; PMCID: PMC11127123.
- "Implications: E-cigarettes may provide a reduced-harm alternative to cigarettes for smokers unwilling/unable to quit or serve as a path for quitting all nicotine products. Higher nicotine concentrations and flavor variety are associated with higher abuse potential and appeal of e-cigarettes. Higher abuse potential and appeal products may help facilitate complete switching from cigarettes to e-cigarettes. Regulation of nicotine concentration and flavors aimed at decreasing naïve uptake may inadvertently decrease uptake and complete switching among smokers, reducing the harm reduction potential of e-cigarettes. Evidence-based effects of regulating nicotine concentration and flavors must be considered for the population as a whole, including smokers."
- Citation: Gades MS, Alcheva A, Riegelman AL, Hatsukami DK. The Role of Nicotine and Flavor in the Abuse Potential and Appeal of Electronic Cigarettes for Adult Current and Former Cigarette and Electronic Cigarette Users: A Systematic Review. Nicotine Tob Res. 2022 Aug 6;24(9):1332-1343. doi: 10.1093/ntr/ntac073. PMID: 35305014; PMCID: PMC9356694.
- Acknowledgment: This work was supported by the National Institute of Drug Abuse (T32 DA007097 and R36 DA050000 to MSG); and the National Institutes of Health (P01 CA217806 to DKH). No COI declared.
- Citation: Gades MS, Alcheva A, Riegelman AL, Hatsukami DK. The Role of Nicotine and Flavor in the Abuse Potential and Appeal of Electronic Cigarettes for Adult Current and Former Cigarette and Electronic Cigarette Users: A Systematic Review. Nicotine Tob Res. 2022 Aug 6;24(9):1332-1343. doi: 10.1093/ntr/ntac073. PMID: 35305014; PMCID: PMC9356694.
- "Results: A total of 494 subjects provided complete data on both surveys. At baseline, 402 subjects (81.4%) were exclusive e-cigarette users, and 71 subjects (14.4%) were poly users. Among baseline exclusive e-cigarette users, the majority (88.3%) continued using e-cigarettes exclusively, but 37 users (9.2%) became poly users and 1 returned to cigarette smoking at follow-up. Among baseline poly users, 60.6% became exclusive e-cigarette users at follow-up. The mean PSECDI score remained similar over time (8.4 at baseline vs 8.3 at follow-up)."
- NOTE: For information on the Penn State Ecigarette Dependence Index see this link. Scores: 4 to 8 is low dependence and 9 to 12 is a medium dependence.
- Citation: Du P, Fan T, Yingst J, Veldheer S, Hrabovsky S, Chen C, Foulds J. Changes in E-Cigarette Use Behaviors and Dependence in Long-term E-Cigarette Users. Am J Prev Med. 2019 Sep;57(3):374-383. doi: 10.1016/j.amepre.2019.04.021. Epub 2019 Jul 31. PMID: 31375364; PMCID: PMC9811611.
- Acknowledgment: This work was supported by the National Institute on Drug Abuse of NIH and the Center for Tobacco Products of the U.S. Food and Drug Administration (P50-DA-036107). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the U.S. Food and Drug Administration. Jonathan Foulds has acted as a paid consultant for pharmaceutical companies involved in producing smoking-cessation medications, including GSK, Pfizer, Novartis, and J&J and received a research grant and study products from Pfizer Inc. No other financial disclosures were reported.
- Citation: Du P, Fan T, Yingst J, Veldheer S, Hrabovsky S, Chen C, Foulds J. Changes in E-Cigarette Use Behaviors and Dependence in Long-term E-Cigarette Users. Am J Prev Med. 2019 Sep;57(3):374-383. doi: 10.1016/j.amepre.2019.04.021. Epub 2019 Jul 31. PMID: 31375364; PMCID: PMC9811611.
- Conclusion: "From a public health perspective, there is a trade-off between e-cigarettes that provide high levels of nicotine, high satisfaction and more effects on craving for tobacco, but may also be addictive, and e-cigarettes that contain less nicotine and are less addictive, but are also less satisfactory and less efficient at relieving craving and at helping dependent smokers quit smoking. This trade-off must be kept in mind when regulating e-cigarettes."
- Citation: Etter JF. Explaining the effects of electronic cigarettes on craving for tobacco in recent quitters. Drug Alcohol Depend. 2015 Mar 1;148:102-8. doi: 10.1016/j.drugalcdep.2014.12.030. Epub 2015 Jan 3. PMID: 25592454.
- Acknowledgment: This study was partly funded by the Swiss Tobacco Prevention Fund (Swiss Federal Office of Public Health), grant 12.000189 to JFE. The Swiss Tobacco Prevention Fund had no role in the design or conduct of the study, interpretation of the data or decision to submit the paper for publication... JFE was reimbursed by Dekang, a manufacturer of e-cigarettes and e-liquids for traveling to London and to China, to visit e-cigarette factories, but he received no honoraria for these meetings. JFE's salary is paid by the University of Geneva... Vincent Baujard, from the HON Foundation, Geneva, Switzerland developed the software for data collection. Thomas Eissenberg (Virginia Commonwealth University, USA), was a consultant for this study...
- Citation: Etter JF. Explaining the effects of electronic cigarettes on craving for tobacco in recent quitters. Drug Alcohol Depend. 2015 Mar 1;148:102-8. doi: 10.1016/j.drugalcdep.2014.12.030. Epub 2015 Jan 3. PMID: 25592454.
Heated Tobacco Product (HTP)
- "These findings suggest that the abuse liability of the THPs lies between that of subjects usual brand cigarettes and the NRT."
- Citation: Hardie G, Gale N, McEwan M, Oscar SM, Ziviani L, Proctor CJ, Murphy J. An abuse liability assessment of the glo tobacco heating product in comparison to combustible cigarettes and nicotine replacement therapy. Sci Rep. 2022 Aug 29;12(1):14701. doi: 10.1038/s41598-022-19167-8. Erratum in: Sci Rep. 2023 Jun 27;13(1):10441. doi: 10.1038/s41598-023-37432-2. PMID: 36038580; PMCID: PMC9424205.
- Acknowledgment: The study was funded in full by British American Tobacco (Investments) Limited (BAT). GH, NG, and MMcE are current employees of BAT. JM was an employee of BAT at the time of the study conduct and is currently an employee of R. J. Reynolds Tobacco Company, a subsidiary of BAT. CJP was an employee of BAT at the time of study conduct and is currently contracted to BAT to provide consultancy on tobacco product science and regulation. SM and LZ are employees of CRC, the clinic who performed the trial.
- Citation: Hardie G, Gale N, McEwan M, Oscar SM, Ziviani L, Proctor CJ, Murphy J. An abuse liability assessment of the glo tobacco heating product in comparison to combustible cigarettes and nicotine replacement therapy. Sci Rep. 2022 Aug 29;12(1):14701. doi: 10.1038/s41598-022-19167-8. Erratum in: Sci Rep. 2023 Jun 27;13(1):10441. doi: 10.1038/s41598-023-37432-2. PMID: 36038580; PMCID: PMC9424205.
Nicotine Pouches
- "Implications: Little is known about the effects of racemic (vs. S-) nicotine in humans. In a sample of adults who smoke cigarettes, we identified that oral nicotine pouches containing racemic nicotine exposed participants to less nicotine than oral nicotine pouches containing only S-nicotine, but both types of oral nicotine pouches held similar, moderate appeal. Additional research evaluating the roles that flavorings, total nicotine concentration, and freebase nicotine play in the abuse liability of oral nicotine pouches would inform comprehensive product regulations to support public health."
- Citation: Keller-Hamilton B, Curran H, Alalwan M, Hinton A, Brinkman MC, El-Hellani A, Wagener TL, Chrzan K, Atkinson L, Suraapaneni S, Mays D. Evaluating the Role of Nicotine Stereoisomer on Nicotine Pouch Abuse Liability: A Randomized Crossover Trial. Nicotine Tob Res. 2024 May 7:ntae079. doi: 10.1093/ntr/ntae079. Epub ahead of print. PMID: 38713545.
- Acknowledgment: Grants: U54CA287392/DA/NIDA NIH HHS/United States, CA/NCI NIH HHS/United States
- Citation: Keller-Hamilton B, Curran H, Alalwan M, Hinton A, Brinkman MC, El-Hellani A, Wagener TL, Chrzan K, Atkinson L, Suraapaneni S, Mays D. Evaluating the Role of Nicotine Stereoisomer on Nicotine Pouch Abuse Liability: A Randomized Crossover Trial. Nicotine Tob Res. 2024 May 7:ntae079. doi: 10.1093/ntr/ntae079. Epub ahead of print. PMID: 38713545.
Heated Tobacco Product (HTP)
- "These findings suggest that the abuse liability of the THPs lies between that of subjects usual brand cigarettes and the NRT."
- Citation: Hardie G, Gale N, McEwan M, Oscar SM, Ziviani L, Proctor CJ, Murphy J. An abuse liability assessment of the glo tobacco heating product in comparison to combustible cigarettes and nicotine replacement therapy. Sci Rep. 2022 Aug 29;12(1):14701. doi: 10.1038/s41598-022-19167-8. Erratum in: Sci Rep. 2023 Jun 27;13(1):10441. doi: 10.1038/s41598-023-37432-2. PMID: 36038580; PMCID: PMC9424205.
- Acknowledgment: The study was funded in full by British American Tobacco (Investments) Limited (BAT). GH, NG, and MMcE are current employees of BAT. JM was an employee of BAT at the time of the study conduct and is currently an employee of R. J. Reynolds Tobacco Company, a subsidiary of BAT. CJP was an employee of BAT at the time of study conduct and is currently contracted to BAT to provide consultancy on tobacco product science and regulation. SM and LZ are employees of CRC, the clinic who performed the trial.
- Citation: Hardie G, Gale N, McEwan M, Oscar SM, Ziviani L, Proctor CJ, Murphy J. An abuse liability assessment of the glo tobacco heating product in comparison to combustible cigarettes and nicotine replacement therapy. Sci Rep. 2022 Aug 29;12(1):14701. doi: 10.1038/s41598-022-19167-8. Erratum in: Sci Rep. 2023 Jun 27;13(1):10441. doi: 10.1038/s41598-023-37432-2. PMID: 36038580; PMCID: PMC9424205.
Nicotine Replacement Therapy (NRT)
- "These data reinforce previous research and provide the scientific evidence to support regulatory decisions demonstrating that Vuse Solo has an AL profile lower than that of combustible cigarettes but higher than that of nicotine gum and, therefore, may be a suitable replacement for cigarette smoking for some adult smokers."
- Citation: Campbell C, Jin T, Round EK, Schmidt E, Nelson P, Baxter S. Part one: abuse liability of Vuse Solo (G2) electronic nicotine delivery system relative to combustible cigarettes and nicotine gum. Sci Rep. 2022 Dec 21;12(1):22080. doi: 10.1038/s41598-022-26417-2. PMID: 36543869; PMCID: PMC9772348.
- Acknowledgment: C.C., T.J., E.S., E.R., and S.B. are full-time employees of RAI Services Company, and P.N. is a former full-time employee of RAI Services Company. RAI Services Company is a wholly owned subsidiary of Reynolds American Inc., which is an indirect, wholly owned subsidiary of British American Tobacco plc.
- Citation: Campbell C, Jin T, Round EK, Schmidt E, Nelson P, Baxter S. Part one: abuse liability of Vuse Solo (G2) electronic nicotine delivery system relative to combustible cigarettes and nicotine gum. Sci Rep. 2022 Dec 21;12(1):22080. doi: 10.1038/s41598-022-26417-2. PMID: 36543869; PMCID: PMC9772348.
- "These findings suggest that the abuse liability of the THPs lies between that of subjects usual brand cigarettes and the NRT."
- Citation: Hardie G, Gale N, McEwan M, Oscar SM, Ziviani L, Proctor CJ, Murphy J. An abuse liability assessment of the glo tobacco heating product in comparison to combustible cigarettes and nicotine replacement therapy. Sci Rep. 2022 Aug 29;12(1):14701. doi: 10.1038/s41598-022-19167-8. Erratum in: Sci Rep. 2023 Jun 27;13(1):10441. doi: 10.1038/s41598-023-37432-2. PMID: 36038580; PMCID: PMC9424205.
- Acknowledgment: The study was funded in full by British American Tobacco (Investments) Limited (BAT). GH, NG, and MMcE are current employees of BAT. JM was an employee of BAT at the time of the study conduct and is currently an employee of R. J. Reynolds Tobacco Company, a subsidiary of BAT. CJP was an employee of BAT at the time of study conduct and is currently contracted to BAT to provide consultancy on tobacco product science and regulation. SM and LZ are employees of CRC, the clinic who performed the trial.
- Citation: Hardie G, Gale N, McEwan M, Oscar SM, Ziviani L, Proctor CJ, Murphy J. An abuse liability assessment of the glo tobacco heating product in comparison to combustible cigarettes and nicotine replacement therapy. Sci Rep. 2022 Aug 29;12(1):14701. doi: 10.1038/s41598-022-19167-8. Erratum in: Sci Rep. 2023 Jun 27;13(1):10441. doi: 10.1038/s41598-023-37432-2. PMID: 36038580; PMCID: PMC9424205.
- Conclusions: Controlled use of JS among adult smokers resulted in nicotine delivery, product liking, and satisfaction that were less than that of combustible cigarettes but generally greater than nicotine gum. These results support the conclusion that JS has lower abuse liability than combustible cigarettes, higher abuse liability than nicotine gum, and may provide sufficient nicotine delivery and satisfying effects to support substitution for combustible cigarettes among adult smokers.
- Citation: Goldenson NI, Buchhalter AR, Augustson EM, Rubinstein ML, Henningfield JE. Abuse liability assessment of the JUUL system in four flavors relative to combustible cigarette, nicotine gum and a comparator electronic nicotine delivery system among adult smokers. Drug Alcohol Depend. 2020 Dec 1;217:108395. doi: 10.1016/j.drugalcdep.2020.108395. Epub 2020 Nov 4. PMID: 33176942.
- Acknowledgment: The study was funded by Juul Labs, Inc. NIG, EMA and MLR are full-time employees of Juul Labs, Inc., JEH and ARB are full-time employees of PinneyAssociates, Inc. PinneyAssociates provides consulting services on tobacco harm reduction on an exclusive basis to Juul Labs, Inc. Within the last two years, PinneyAssociates has consulted for British American Tobacco and Reynolds American Inc and subsidiaries on tobacco harm reduction. JEH co-holds a patent for a novel nicotine medication that has not been developed or commercialized. There are no other interests declared by authors.
- Citation: Goldenson NI, Buchhalter AR, Augustson EM, Rubinstein ML, Henningfield JE. Abuse liability assessment of the JUUL system in four flavors relative to combustible cigarette, nicotine gum and a comparator electronic nicotine delivery system among adult smokers. Drug Alcohol Depend. 2020 Dec 1;217:108395. doi: 10.1016/j.drugalcdep.2020.108395. Epub 2020 Nov 4. PMID: 33176942.
- Conclusions: These results suggest that the abuse liability of both 5.0 % and 3.0 % JS is: (1) substantially lower than UB cigarette; (2) somewhat lower than comparator ENDS; and (3) higher than nicotine gum. Additionally, the abuse liability of JS 5.0 % is somewhat higher than JS 3.0 %.
- Citation: Goldenson NI, Buchhalter AR, Augustson EM, Rubinstein ML, Van Hoof D, Henningfield JE. Abuse liability assessment of the JUUL system in two nicotine concentrations compared to combustible cigarette, nicotine gum and comparator electronic nicotine delivery system. Drug Alcohol Depend. 2020 Dec 1;217:108441. doi: 10.1016/j.drugalcdep.2020.108441. Epub 2020 Nov 24. PMID: 33250386.
- Acknowledgment: The study was funded by Juul Labs, Inc. NIG, EMA, DVH and MLR are full-time employees of Juul Labs, Inc., JEH and ARB are full-time employees of PinneyAssociates, Inc. PinneyAssociates provides consulting services on tobacco harm reduction on an exclusive basis to Juul Labs, Inc. Within the last two years, PinneyAssociates has consulted for British American Tobacco and Reynolds American Inc and subsidiaries on tobacco harm reduction. JEH co-holds a patent for a novel nicotine medication that has not been developed or commercialized. There are no other interests declared by authors.
- Citation: Goldenson NI, Buchhalter AR, Augustson EM, Rubinstein ML, Van Hoof D, Henningfield JE. Abuse liability assessment of the JUUL system in two nicotine concentrations compared to combustible cigarette, nicotine gum and comparator electronic nicotine delivery system. Drug Alcohol Depend. 2020 Dec 1;217:108441. doi: 10.1016/j.drugalcdep.2020.108441. Epub 2020 Nov 24. PMID: 33250386.
- "Collectively, the results of this study demonstrated that the ECIG device and liquids examined had moderate levels of abuse liability: on average lower than combustible cigarettes, but higher than an FDA-approved nicotine replacement therapy (i.e., nicotine inhaler)."
- Citation: Maloney SF, Breland A, Soule EK, Hiler M, Ramôa C, Lipato T, Eissenberg T. Abuse liability assessment of an electronic cigarette in combustible cigarette smokers. Exp Clin Psychopharmacol. 2019 Oct;27(5):443-454. doi: 10.1037/pha0000261. Epub 2019 Feb 18. PMID: 30777773; PMCID: PMC6754311.
- Acknowledgment: Funding; This study was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number P50DA036105 and U54DA036105 and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration. COI; Dr. Eissenberg is a paid consultant in litigation against the tobacco industry and is named on a patent for a device that measures the puffing behavior of ECIG users.
- Citation: Maloney SF, Breland A, Soule EK, Hiler M, Ramôa C, Lipato T, Eissenberg T. Abuse liability assessment of an electronic cigarette in combustible cigarette smokers. Exp Clin Psychopharmacol. 2019 Oct;27(5):443-454. doi: 10.1037/pha0000261. Epub 2019 Feb 18. PMID: 30777773; PMCID: PMC6754311.
- These findings are concordant with our previous results and provide evidence that menthol Vuse Solo ECs have abuse liability that is lower than menthol cigarettes and potentially greater than that of nicotine gum.
- PDF Version
- Citation: Stiles MF, Campbell LR, Jin T, Graff DW, Fant RV, Henningfield JE. Assessment of the abuse liability of three menthol Vuse Solo electronic cigarettes relative to combustible cigarettes and nicotine gum. Psychopharmacology (Berl). 2018 Jul;235(7):2077-2086. doi: 10.1007/s00213-018-4904-x. Epub 2018 May 3. PMID: 29725702; PMCID: PMC6015619.
- Acknowledgement: ClinicalTrials.gov identifier: NCT02664012. MF Stiles, LR Campbell, and T Jin are full-time employees of RAI Services Company. RAI Services Company is a wholly owned subsidiary of Reynolds American Inc., which is a wholly owned subsidiary of British American Tobacco plc. DW Graff is a full-time employee of Celerion and provided the original draft of the manuscript. RV Fant and JE Henningfield are full-time employees of PinneyAssociates, which provides consulting services on smoking cessation and tobacco harm minimization (including nicotine replacement therapy and electronic vapor products) to Niconovum, USA, Inc., RJ Reynolds Vapor Company, and RAI Services Company (all subsidiaries of Reynolds American Inc.). JE Henningfield also owns an interest in intellectual property for a novel nicotine medication. Through PinneyAssociates, Fant and Henningfield provide consulting services to pharmaceutical companies on abuse potential assessment, and the regulation of substances with a potential for abuse.
- Citation: Stiles MF, Campbell LR, Jin T, Graff DW, Fant RV, Henningfield JE. Assessment of the abuse liability of three menthol Vuse Solo electronic cigarettes relative to combustible cigarettes and nicotine gum. Psychopharmacology (Berl). 2018 Jul;235(7):2077-2086. doi: 10.1007/s00213-018-4904-x. Epub 2018 May 3. PMID: 29725702; PMCID: PMC6015619.
- "In summary, this study is the most robust assessment of the abuse liability of ECs published to date and uses approaches similar to those found in classic abuse liability studies of pharmaceutical products, including multiple instruments to measure the subjective effects of product use, as well as nicotine uptake. Under the set of study conditions described herein, use of the three Vuse Solo ECs tended to result in subjective measures responses and nicotine uptake that were between those measured with use of combustible cigarettes and nicotine gum. In general, the results are consistent with the conclusions of others that the abuse liability of ECs as a category is less than that of combustible cigarettes but greater than for nicotine gum, and likely other nicotine replacement products'
- Citation: Stiles MF, Campbell LR, Graff DW, Jones BA, Fant RV, Henningfield JE. Pharmacodynamic and pharmacokinetic assessment of electronic cigarettes, combustible cigarettes, and nicotine gum: implications for abuse liability. Psychopharmacology (Berl). 2017 Sep;234(17):2643-2655. doi: 10.1007/s00213-017-4665-y. Epub 2017 Jun 20. PMID: 28634710; PMCID: PMC5548902.
- Acknowledgment: Funding; This study was funded by RJ Reynolds Vapor Company through its affiliate RJ Reynolds Tobacco Company. COI; MF Stiles, LR Campbell, and BA Jones are full-time employees of RAI Services Company, which provides support across the Reynolds American Inc. operating companies. DW Graff is a full-time employee of Celerion and provided the original draft of this manuscript. RV Fant and JE Henningfield are full-time employees of PinneyAssociates, which provides consulting services on tobacco harm minimization (including nicotine replacement therapy and digital vapor products) to Niconovum USA, RJ Reynolds Vapor Company, and RAI Services Company (all subsidiaries of Reynolds American Inc.) In the past 3 years, PinneyAssociates has consulted to GlaxoSmithKline Consumer Healthcare on smoking cessation and NJOY on electronic cigarettes. JE Henningfield also owns an interest in intellectual property for a novel nicotine medication, an option for which has been sold to Niconovum USA. Through PinneyAssociates, Fant and Henningfield also provide consulting services to pharmaceutical companies on abuse potential assessment and the regulation of substances with a potential for abuse.
- Citation: Stiles MF, Campbell LR, Graff DW, Jones BA, Fant RV, Henningfield JE. Pharmacodynamic and pharmacokinetic assessment of electronic cigarettes, combustible cigarettes, and nicotine gum: implications for abuse liability. Psychopharmacology (Berl). 2017 Sep;234(17):2643-2655. doi: 10.1007/s00213-017-4665-y. Epub 2017 Jun 20. PMID: 28634710; PMCID: PMC5548902.
- Conclusion: "Conclusions: Some e-cigarette users were dependent on nicotine-containing e-cigarettes, but these products were less addictive than tobacco cigarettes."
- Citation: Etter JF, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015 Feb 1;147:68-75. doi: 10.1016/j.drugalcdep.2014.12.007. Epub 2014 Dec 18. PMID: 25561385; PMCID: PMC4920051.
- This study was partly funded by the Swiss Tobacco Prevention Fund (Swiss Federal Office of Public Health), grant 12.000189 to JFE. The Swiss Tobacco Prevention Fund had no role in the design or conduct of the study, interpretation of the data or decision to submit the paper for publication. TE is supported by the National Institute on Drug Abuse of the U.S. National Institutes of Health under Award Number P50DA036105 and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
- Citation: Etter JF, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015 Feb 1;147:68-75. doi: 10.1016/j.drugalcdep.2014.12.007. Epub 2014 Dec 18. PMID: 25561385; PMCID: PMC4920051.
2013 Modifications To Labeling of Nicotine Replacement Therapy Products for Over-the-Counter Human Use
- We also note that although any nicotine-containing product has the potential to be addicting, based on the available evidence, currently marketed OTC NRT products do not appear to have significant potential for abuse or dependence. A 2010 review of historical reports made to the Agency's Adverse Event Reporting System and to the Substance Abuse and Mental Health Services Administration's Drug Abuse Warning Network between 1984 and 2009 suggested that NRT products have a low potential for abuse. Several published studies have also found that the abuse liability and dependence potential of NRT products is low, especially compared to cigarettes.
- PDF Version
- Citation: Food and Drug Administration, 78 FR 19718
- Results suggest that the nicotine lozenge has low abuse liability, both in adults and young adults.
- PDF Version
- Citation: Houtsmuller EJ, Henningfield JE, Stitzer ML. Subjective effects of the nicotine lozenge: assessment of abuse liability. Psychopharmacology (Berl). 2003 Apr;167(1):20-7. doi: 10.1007/s00213-002-1361-2. Epub 2003 Mar 4. PMID: 12616334.
- Acknowledgement: This research was supported by SmithKline Beecham Consumer Healthcare.
- Citation: Houtsmuller EJ, Henningfield JE, Stitzer ML. Subjective effects of the nicotine lozenge: assessment of abuse liability. Psychopharmacology (Berl). 2003 Apr;167(1):20-7. doi: 10.1007/s00213-002-1361-2. Epub 2003 Mar 4. PMID: 12616334.
- Mint-flavored nicotine gum was rated as more palatable than the original nicotine gum, but the improvement in flavor did not increase abuse liability in adults (22 – 50 years old) or young adults (18 –21 years old).
- PDF Version
- Citation: Houtsmuller EJ, Fant RV, Eissenberg TE, Henningfield JE, Stitzer ML. Flavor improvement does not increase abuse liability of nicotine chewing gum. Pharmacol Biochem Behav. 2002 Jun;72(3):559-68. doi: 10.1016/s0091-3057(02)00723-2. PMID: 12175452.
- Acknowledgement: This study was supported by SmithKline Beecham Consumer Healthcare.
- Citation: Houtsmuller EJ, Fant RV, Eissenberg TE, Henningfield JE, Stitzer ML. Flavor improvement does not increase abuse liability of nicotine chewing gum. Pharmacol Biochem Behav. 2002 Jun;72(3):559-68. doi: 10.1016/s0091-3057(02)00723-2. PMID: 12175452.
- "We conclude that abuse liability from all four NRT products was low. Subjective dependence was moderate and did not differ across products. Behavioural dependence was modest and was positively related to rate of nicotine delivery."
- Citation: West R, Hajek P, Foulds J, Nilsson F, May S, Meadows A. A comparison of the abuse liability and dependence potential of nicotine patch, gum, spray and inhaler. Psychopharmacology (Berl). 2000 Apr;149(3):198-202. doi: 10.1007/s002130000382. PMID: 10823399.
- Acknowledgment: This study was funded by Pharmacia and Upjohn, Sweden.
- Citation: West R, Hajek P, Foulds J, Nilsson F, May S, Meadows A. A comparison of the abuse liability and dependence potential of nicotine patch, gum, spray and inhaler. Psychopharmacology (Berl). 2000 Apr;149(3):198-202. doi: 10.1007/s002130000382. PMID: 10823399.
- "Overall, results are consistent with the conclusion that the nicotine nasal spray and vapor inhaler are of substantially lower abuse liability than cigarettes in experienced cigarette smokers receiving initial exposure to these products."
- Citation: Schuh KJ, Schuh LM, Henningfield JE, Stitzer ML. Nicotine nasal spray and vapor inhaler: abuse liability assessment. Psychopharmacology (Berl). 1997 Apr;130(4):352-61. doi: 10.1007/s002130050250. PMID: 9160851.
- Acknowledgment: This work was supported by USPHS research grant DA03893 and training grant T32 DA07209 from the National Institute on Drug Abuse and conducted in collaboration with the NIDA Intramural Research Program Addiction Research Center. The authors thank Pharmacia Upjohn who kindly donated pharmaceutical supplies and conducted blood assays for this study.
- Citation: Schuh KJ, Schuh LM, Henningfield JE, Stitzer ML. Nicotine nasal spray and vapor inhaler: abuse liability assessment. Psychopharmacology (Berl). 1997 Apr;130(4):352-61. doi: 10.1007/s002130050250. PMID: 9160851.
Novel Oral Products (Chewable)
- "The test products, under the conditions of this study, carry lower abuse potential than own-brand cigarettes and similar to nicotine polacrilex gum."
- Citation: Liu J, Wang J, Vansickel A, Edmiston J, Graff D, Sarkar M. Characterization of the Abuse Potential in Adult Smokers of a Novel Oral Tobacco Product Relative to Combustible Cigarettes and Nicotine Polacrilex Gum. Clin Pharmacol Drug Dev. 2021 Mar;10(3):241-250. doi: 10.1002/cpdd.909. Epub 2021 Jan 27. PMID: 33502815; PMCID: PMC7986766.
- Acknowledgment: J.L., J.W., A.V., J.E., and M.S. are employees of Altria Client Services LLC. D.G. was an employee of Celerion, Inc., who was contracted by Altria Client Services LLC to perform the study and analyze the study data.
- Citation: Liu J, Wang J, Vansickel A, Edmiston J, Graff D, Sarkar M. Characterization of the Abuse Potential in Adult Smokers of a Novel Oral Tobacco Product Relative to Combustible Cigarettes and Nicotine Polacrilex Gum. Clin Pharmacol Drug Dev. 2021 Mar;10(3):241-250. doi: 10.1002/cpdd.909. Epub 2021 Jan 27. PMID: 33502815; PMCID: PMC7986766.
VLNC - Very Low Nicotine Cigarettes
- WARNING! It is still smoking and has all the harms of smoking, even if less addictive.
- 'In the overall population, 4.1% reported that low-nicotine cigarettes were much more addictive than typical cigarettes, 67.5% said they were equally addictive, while 28.4% reported they were slightly/much less addictive."
- Citation:
- Acknowledgement:
- Citation:
2021: Reactions to reduced nicotine content cigarettes in a sample of young adult, low-frequency smokers
- Reducing nicotine content will likely lower the abuse liability of cigarettes for most young, low-frequency smokers. Additional work is needed to determine if compensatory smoking may lead to increased toxicant exposure, and if a subset of individuals choosing lower nicotine cigarettes may continue to smoke regardless of nicotine content.
- Citation:
- Acknowledgement:
- Citation:
- Several participants expected, prior to trying VLNC cigarettes, to compensate for the reduced nicotine levels by smoking more cigarettes but were surprised when they did not increase their smoking. A subset of participants reported experiencing minor withdrawal symptoms, such as irritability and fatigue. Several participants reported feeling less dependent after exclusively smoking VLNC cigarettes. Most participants said they would smoke VLNC cigarettes if they were the only cigarettes available to purchase. Some also said that smoking VLNC cigarettes could help people taper down or quit smoking.
- Citation:
- Acknowledgement:
- Citation:
- These 3 randomized clinical trials including 775 participants with affective disorders, opioid use disorder, or socioeconomic disadvantage found that reducing nicotine content significantly decreased total cigarettes smoked daily and nicotine dependence severity.
- Citation:
- Acknowledgement:
- Citation:
- Ten pregnant smokers in Burlington, VT and Baltimore, MD participated in 2017–2018.
- Reducing the nicotine content of cigarettes may decrease their abuse liability in pregnant smokers without causing untoward craving/withdrawal or compensatory smoking. Studies of extended exposure to VLNCs in pregnant women are warranted.
- Citation:
- Acknowledgement:
- Citation:
- In this 6-week study, reduced-nicotine cigarettes versus standard-nicotine cigarettes reduced nicotine exposure and dependence and the number of cigarettes smoked.
- Citation:
- Acknowledgement:
- Citation:
- "Unlike the 0.3 mg cigarettes, 0.05 mg cigarettes were not associated with compensatory smoking behaviors. Furthermore, the 0.05 mg cigarettes and nicotine lozenge were associated with reduced carcinogen exposure, nicotine dependence and product withdrawal scores. The 0.05 mg cigarette was associated with greater relief of withdrawal from usual brand cigarettes than the nicotine lozenge. The 0.05 mg cigarette led to a significantly higher rate of cessation than the 0.3 mg cigarette and a similar rate as nicotine lozenge."
- Citation:
- Acknowledgement:
- Citation:
Suggestions to add to this page
- Sharon Cox