UserWiki:Richardpruen
| Babel user information | ||
|---|---|---|
| ||
| Users by language |
The Site admin
Richard Pruen
Electrical and electronic engineer
Favorite band: Rush
Why spend so much time on this? It’s a fact that nicotine harm reduction (vaping and Snus) saved my life, this is my way to pay that forward, and pass along information on safer nicotine.
Running a site from the ground up, has proved interesting and worthwhile. Some of the things I have learned about Linux, and configuration of software for servers is only stuff you learn by doing.
I suspect the site will stick around, at least as long as it is required to provide links and info.
I might well put more info of my vaping / thr story here soon, this is just a test edit.
More test edit, and added some features, like babel to indicate users languages.
Site admin at Safer nicotine wiki.

ABOUT
Ecig user since 2008, consumer advocate for vaping and THR #VapingSavedMyLife #VapingSavesLives
Twitter @pruenrichard
Below are letters and documents sent in advocacy efforts as a record and should anyone wish to use them for ideas. These are my efforts and do not necessarily reflect the views of the wiki or other users.







![Response ID ANON-EJTE-W3QH-B Submitted to Proposed reforms to the regulation of vapes Submitted on 2023-09-08 21:15:44 Survey contents Privacy and your personal information I consent to the TGA collecting the information requested in this survey about me, including any sensitive information, for the purposes indicated above. Agree: Yes Acknowledgement I agree. Yes.: Yes Introduction 1 What is your name? Name: Richard Pruen 2 What is your email address? Email: richard@pruen.co.uk 3 What is your organisation name? Organisation name or N/A: n/a 4 Please choose a stakeholder group that best describes you or your organisation. Others* *If other, please specify:: Consumer 5 Which best describes your response? I am responding as an individual. 6 Are you an authorised prescriber? No (please go to next page Conflicts of interest (actual or perceived) 1 Have you or your organisation ever received services, assistance or support (whether monetary or non-monetary in nature) from the tobacco industry and/or e-cigarette industry? If this scenario applies to you or your organisation, please provide relevant details in the textbox. Yes If you have selected yes, please provide details here. Otherwise, please state 'Not Applicable':: I owned a company testing electronic cigarettes for compliance with UK and other standards, as well as carried out investigations of failures including battery failure. I also worked on R&D and provided consulting services on for example battery protection circuits, chargers etc. Payment was from user fees for testing, government agencies (trading standards), trade associations, and sometimes individuals or companies carrying out due diligence testing or failure mode analysis. Ended 2016 since then I have been a consumer only and have not received any funding from any company (of any sort), government agency, or researchinstitution. 2 Have you or your organisation ever provided services, assistance or support (whether monetary or non-monetary in nature) to the tobacco industry and/or the e-cigarette industry? If this scenario applies to you or your organisation, please provide further information in the textbox. Yes If you have selected 'yes', please provide details here. Otherwise, please state 'Not Applicable': I assisted in writing standards for UK vaping products (PAS 54115 A guide for the importation and sale of electronic cigarettes and directly related products, with product safety testing methods) and proposed an IEEE standard project number P2800. Several sampling machines were supplied to others testing electronic cigarettes, and other custom test equipment, the supplied equipment presumably used to carry out tests on electronic cigarettes as designed. As academic and test houses purchased this equipment it is assumed this was paid for by test fees, or grants to carry out tests on electronic cigarettes, again possibly linked to taxes etc from tobacco and or electronic cigarette sales. The same funding source as government agencies, politicians and others working in the field. Again since 2016 I have been a consumer only. Proposal 1 -Restrictions on importation, manufacture and supply of all vapes . 1 Do you support the proposed approach to ban disposable single use vapes absolutely and all other vapes, except those for legitimate therapeutic use in compliance with the TG Act? No 2 How would you anticipate industry and consumers to respond to a ban on the importation, manufacture and supply of non-therapeutic vapes? * Please provide answer here. : Demand will remain, along with the risk of vaping being seen as cool because it is something disallowed. The tightening of rules is unlikely to be enforceable, so illicit supply will continue to be the primary supply to the population. The population know vaping is safer than smoking, thus it will be very difficult to curb demand. Prohibiting drugs such as cocaine and cannabis fails, vaping prohibition will almost certainly follow the same pattern. 3 Do you support removal of the personal importation scheme exception for vapes? If not, what would be the impact on you? No (* if not, what would be the impact on you?) * What would be the impact on you?: Vaping products that are acceptable and usable are likely to only be available via illicit sales, and criminal gangs. Particularly as research shows flavours to be vital for the prevention of release to smoking. These products will not be tested or regulated in any way, importing allows users who have a prescription to import what they need to stay smoke-free, thus not relapse to smoking and potentially death. Importantly importing from somewhere with a regulated legal market, they can be sure of the quality and contents of the products they buy. The one-size-fits-all approved vapes that consumers will find at pharmacy outlets is unlikely to be effective since most of the features that make an effective substitute for cigarettes will be regulated out. 4 Do you agree with the proposal to retain a traveller’s exemption, including the proposed limits? Yes 5 Do you support the proposed approach to prohibiting the advertisement of all vapes (subject to limited exceptions)? No 6 [If applicable] Suppliers, what part of supply chain do you occupy? Not applicable * Other -specify your role in supply chain.: Consumer 6 (a) What proportion of your sales volumes is attributable to vape sales [i.e. quantity of vapes sold]? Please provide details here: (or mark Not applicable).: N/A6 (b) What proportion of your sales revenue is attributable to vape sales [i.e. revenue earned from sales]? Please provide details here: (or mark Not applicable).: N/A 6 (c) What impact would the proposed measures have on your sales volumes? Please provide details here: (or mark Not applicable).: not a vendor, none 6 (d) What impact would the proposed measures have on your sales revenues? Please provide details here: (or mark Not applicable).: none not a vendor 6 (e) What proportion of your vapes sales is attributable to disposable single use vapes versus refillable products? Please provide details here: (or mark Not applicable).: not a vendor 6 (f) How would restricting the importation, manufacture and supply of disposable single use, and non-therapeutic, vapes in Australia impact you? Please provide details here: (or mark Not applicable).: not a vendor N/A 6 (g) How much stock do you have in Australia currently and how long would it take to sell that stock? Please provide details here: (or mark Not applicable).: not a vendor N/A 6 (h) What would be the cost to you if you were required to dispose or otherwise move on existing stock? Please provide details here: (or mark Not applicable).: N/A Proposal 2 -Changes to market accessibility requirements, including better regulation of device components. 7 Do you support the approach to require a pre-market notification of compliance with TGO 110? No 8 [If applicable] For suppliers of therapeutic vapes, what impact would the proposed notification system have on your supply model and what transition period would you require to comply with the new notification requirement? Please provide details here: (or mark Not applicable).: not a vendor 9 Do you support the proposed access to vapes under the SAS C notification system? No 9 (a) What impact would this pathway have on facilitating patient access to therapeutic vapes? Please provide details here: (or mark Not applicable).: Vaping works in the rest of the world as a product substitute, providing a safer alternative to a deadly (cigarettes) product. In the UK and NZ smoking decline has accelerated significantly past that in Australia, due to the adoption of consumer vaping, slightly less so in the US where the regulator is not so certain. The prescription model in Australia has failed, smoking has not continued to decline and may have increased possibly. Vaping is also higher in Australian youth than UK and NZ, the current policy has backfired and should be reversed. It is absolutely obvious at this point that unless you have unlimited resources for enforcement, the current policy is not workable, and never will be. The time has come to use what has worked elsewhere and start again.10 [If applicable] For prescribers, would the proposed new pathway likely change your approach to prescribing therapeutic vapes? How? Not a prescriber of vapes * How new pathway will change your approach to prescribing ttherapeutic vapes?: 11 [If applicable] For prescribers, which access pathway (SAS B, SAS C, or AP) would you envisage using to prescribe therapeutic vapes? Why? Not a prescriber of vapes Please tell us why: 12 [If applicable] For prescribers, would integration of SAS or AP applications or notifications into existing clinical software systems ease the administrative burden and/or encourage you to use the new pathway? Not a prescriber of vapes 13 Do you agree with the proposal to regulate both e-liquid and device components of unapproved vapes under the same part of the TG Act for simplicity? No 14 Will these changes have direct or indirect impact on you? Please provide details. Yes (please provide details below) Please provide details here:: As a consumer I want safer options to be available to me and to every person who smokes tobacco or would smoke tobacco for lack of a viable alternative. Sadly some kids will do adult things even if you try to stop them (for example drinking alcohol, unsafe sex, taking drugs, smoking) while they should not be encouraged to vape, it still offers a harm reduction if it diverts them from smoking, like it or not, this is true because safer is safer. 15 Do you require time to adjust to these requirements? If yes, how long? Yes 15 (a) How long do you require to adjust to these requirements? More than 12 months Proposal 3 - Improving quality standard for unapproved (unregistered) vapes) 16 Are the definitions of nicotine and mint flavours appropriate? If not, please provide reasons. No (* please provide reason below) * Please provide reason here.: Nicotine itself does not have a flavour, the flavour should be correctly referred to as "artificial tobacco flavour" There is no evidence that human flavour preference varies by age and none particularly that it changes at 18. It is therefore impossible to target age groups with specific flavours, thus limiting flavours on this basis has no merit whatsoever. 17 Do you agree with the proposed upper limit on the concentration of menthol in vapes? If not, please provide reasons. No (* please provide reason below) * Please provide reason here: It is trivial to add menthol to vapes, this simply encourages users to add more if they prefer, opening a can of worms and the potential for contamination or incompatible ingredients. This seem unwise for very little if any benefit. 18 [If applicable] Importers, manufacturers and suppliers, would the restrictions on flavour proposed above impact you? Not applicable 19 Do you agree with the proposal to require pharmaceutical-like packaging and presentation for vapes, e.g., vapes manufactured in black, white or grey coloured materials, predominantly white background on packaging, clear warning statements and other restrictions on labels in addition to other selective TGO 91 requirements for vapes? No (* please provide reason below)20 [If applicable] What impact will the labelling and packaging changes have on you? * Please provide detail here.: It will probably backfire, making legal products undesirable is likely to increase the number of people using illicit products, particularly if access remains easy, without enforcement that is most likely to be the case. 20 (a) How long would you need to transition your product to comply with the proposed requirements? More than 12 months 21 Do you agree with our approach to allow only permitted ingredients in vapes, instead of trying to prohibit individual chemical entities from use in e-liquids? No 22 [If applicable] Importers, manufacturers and suppliers, will your therapeutic vapes need any re-formulation or other changes to comply with the permitted ingredients and ingredient quality requirements? Not applicable 22 (a) If product re-formulation is required, how long will you need to make these changes? More than 12 months 22 (b) If product re-formulation is required, what financial or business impacts would be associated with them? Provide detail here or put 'Not Applicable': Not a vendor 23 Do you support applying the same regulatory controls to zero-nicotine therapeutic vapes, as for NVPs? No 24 What is the overall business cost on you to comply with a strengthened TGO 110? Please provide details here: (or mark Not applicable).: not a vendor 25 Do you agree with the proposed requirements under TGO 110 that will apply to unapproved device components of vapes? No 26 [If applicable] Suppliers, do you intend to register any vaping device on the register as an approved medical device? No (if no, why not?) If no, why not?: not a vendor 27 [If applicable] Importers, manufacturers and suppliers, are you familiar with relevant US FDA, or MHRA guidance and/or EU standards covering vaping devices? Not applicable 27 (a) Do your vapes currently comply with relevant US FDA, or MHRA guidance and/or EU standards covering vaping devices? Not applicable 27 (b) If not, what requirements do you meet? What requirements you currently comply with?: not a vendor 27 (c) How long would it take to achieve compliance with relevant standards? More than 12 months28 [If applicable] Importers, manufacturers and suppliers, are your vapes manufactured at facilities that hold relevant international standards for Quality Management Systems, such as ISO9001 or ISO 13485? Not applicable Proposal 4 - Strengthening domestic compliance and enforcement mechanisms 29 Do you have any other comments in relation to this proposal? Yes (* provide your comments below) Comments: I reiterate that Australian policy has failed, and further moves in the same direction are likely to fail. Other countries have shown much more desirable results, the best results on smoking have been where vaping has been accepted as a consumer alternative to smoking, but the prescription model, unfortunately, has proven unworkable. Realistically you should review the past responses to enquiries, select a number of people who have successfully predicted the results of current regulations, and ask them for solutions. At least they have a track record, and proven knowledge of the real world and likely outcomes. Supplementary questions 30 [If applicable] Suppliers, please confirm if you intend to continue to supply therapeutic vapes under the proposed reforms described? Not applicable * Product range : Not a vendor, but as a consumer, I can see vendors dropping out. This will benefit the criminals and gangs who supply illicit markets, more trade for them. 30 (a) How long would it take to meet the new requirements? More than 12 months 31 [If applicable] Suppliers, please confirm if you intend to register your therapeutic vapes in the next 2 years? Not applicable What guidance and/or clarity of supporting data requirements do you need from TGA: not a vendor Publication of submissions To proceed, please select from the options below how you would like the TGA to deal with your submissions: I agree to the TGA publishing my response in full. I request the TGA to consider redacting sensitive commercial information from my response before publication: No Please specify sensitive commercial inforation you want redatced :](/mediawiki/images/thumb/8/8b/TGA_consult_8-sept-2023_my_response.pdf/page1-300px-TGA_consult_8-sept-2023_my_response.pdf.jpg)



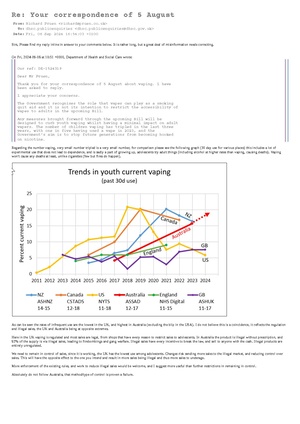
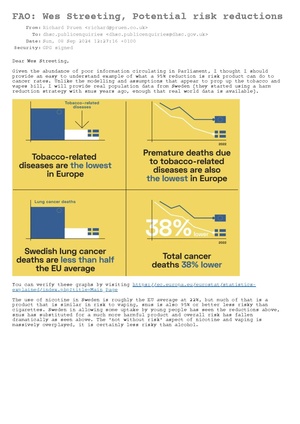
![Subject: COP 10 WHO FCTC Dear Rosanna O’Connor, I am a consumer advocate for vaping, and do not use disposables beyond experimenting to see what they are like. I am also an engineer. I have worked in the vaping industry previously testing compliance with the standards for vaping products, and owned a now closed company BTC Battery Testing LTD (closed 2016). Currently I care for my mother who suffers vascular dementia, and as such volunteer my spare time to the cause of Tobacco Harm Reduction. I believe that consumers should be present at the COP 10 meeting, it is consumers who have the most to gain from stopping the use of combustible tobacco, and the lived experience of trying to do so. Many millions have found a way to eliminate almost all the risk via THR products. The phrase ‘Nothing about us without us’ springs to mind. Why then are the public and press excluded. I would like to see the proceedings live streamed, so those effected can see what is being done. In no way could this effect the discussions, thus is reasonable transparency. I would like an answer regarding the possibility, please. Many countries already found harm reduction useful, critical even. Be that low risk Snus (Sweden has the lowest cancer rate in Europe, and will be smoking free this year (>5%)). Vaping that the UK NHS finds almost 66% effective with support, as stated on their website. New Zealand has seen similar results, especially in native populations, reducing disparities. Japan has seen huge drops in cigarette sales due to reduced harm heated tobacco products. Please see the letter from the Lancet from Robert Beaglehole and Ruth Bonita. They were both senior officials at WHO and are now at the University of Auckland. Robert was formerly Director of the Department of Chronic Disease and Health Promotion at WHO. Ruth was formerly the Director of Surveillance in the Noncommunicable Disease Cluster at WHO. I wish to add my support for their recommendations, the article is attached (also link here https://doi.org/10.1016/S0140-6736(24)00140-5) please make sure these points are discussed at the COP 10 meeting. Yours sincerely, Richard Pruen P.S Please ensure the representatives listed on the next page can discuss before the metting and forward more widely if you agree with the sentiment. Katherine Sands Tobacco Control Team Leader Department for Health and Social Care Martin Dockrell Tobacco Control Programme Lead Department for Health and Social Care Alison Walker Senior Tobacco Control Policy Lead Department of Health and Social Care Esther Lawrence Deputy Head of Global Health UK Mission to the UN, Geneva Please find attached a letter from [THELANCET-D-24-00371] S0140-6736(24)00140-5](/mediawiki/images/thumb/4/4f/Letter_to_cop_10_repersentitives.pdf/page1-300px-Letter_to_cop_10_repersentitives.pdf.jpg)








DRAFT:

